Profile
Dr Pierre Maillard obtained his PhD at the Ecole Polytechnique Fédérale de Lausanne in Switzerland, where he studied a mechanism of cell-intrinsic defence against retroviruses. During his postdoctoral studies, Pierre pursued his work on innate antiviral defences by first studying the antiviral function of RNA interference in mammals in Zürich at the Swiss Federal Institute of Technology and then moved to London to explored the interplay between antiviral RNA interference and the interferon system at the Francis Crick Institute. In 2017, Pierre was awarded a Wolfson UCL Excellence Fellowship to establish his independent career in the Division of Infection and Immunity at University College London. In 2020, Pierre was awarded a UKRI Future Leaders Fellowship and a Barts Charity Lectureship to start his group at Queen Mary University of London within the Blizard institute.
Maillard Lab website
Twitter: @PV_Maillard
ORCID ID: orcid.org/0000-0003-4611-388X
Research
Research Interests:
Research in the Maillard Lab aims to understand the mechanisms by which cells defend themselves against viruses.
All living organisms have evolved mechanisms to defend themselves against viruses. In plants and invertebrates, RNA interference (RNAi) constitutes a major antiviral mechanism, in which long double-stranded RNA (dsRNA) generated during viral replication is cleaved by the enzyme Dicer into small interfering RNAs (siRNAs). These are then loaded onto Argonaute (Ago) proteins to form the RNA-induced silencing complex that recognizes and cleaves complementary viral RNAs to block viral replication. Importantly, the RNAi pathway is conserved in vertebrates and is involved in the generation and function of micro-RNAs (miRNAs), essential for the regulation of gene expression.
However, in vertebrates, long double-stranded RNA is sensed by cytosolic RIG-I like receptors to initiate a signalling cascade that culminates in secretion of interferons (IFN) and expression of hundreds of antiviral IFN-stimulated genes that inhibit virus replication.
Recent evidence has emerged for a functional role of antiviral RNAi in mammals opening-up a new area of research into characterising when, where and how this ancestral antiviral mechanism plays a role in mammals.
The Maillard Lab aims to characterise:
- the cell types and contexts in which RNAi plays a role in the mammalian antiviral defence system
- the cellular mechanisms that regulate the IFN response.
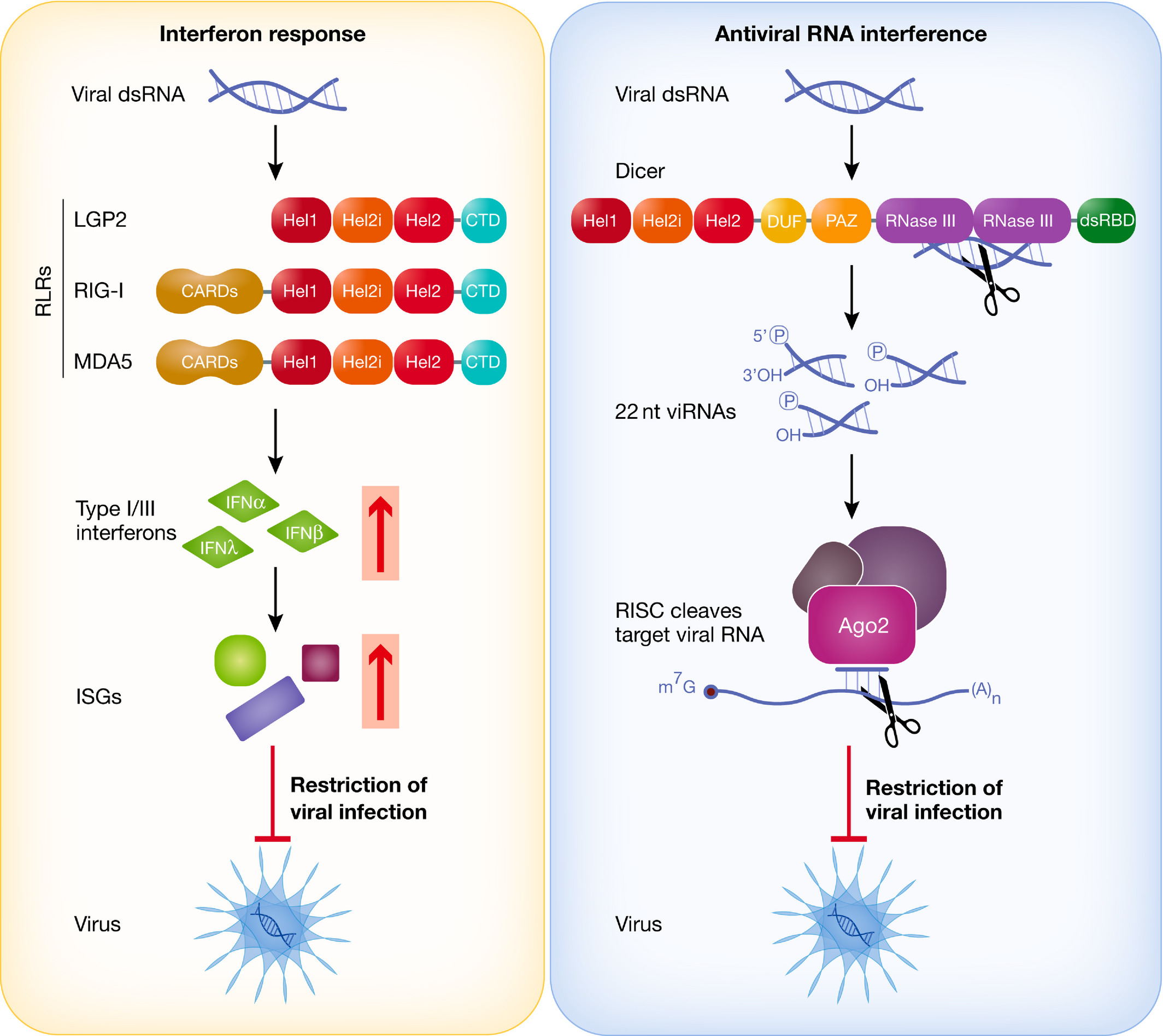
IFN response and antiviral RNAi triggered by viral dsRNA.
In the cytoplasm of mammalian cells, the RIG‐I‐like receptors (RLRs) RIG‐I and MDA5 detect viral dsRNA and trigger the production of type I interferons, which results in the induction of interferon‐stimulated genes (ISGs) that encode proteins capable of inhibiting viral replication and virus spread. In antiviral RNAi, Dicer cleaves viral dsRNA into viRNAs that are loaded into a RISC complex. As a protein component of this complex, Ago2 degrades viral RNAs with homology to the viRNAs, thereby inhibiting viral replication. RLRs and Dicer share a common DExD/H domain, composed of three helicases (Hel1, Hel2i and Hel2). RIG‐I and MDA5 additionally carry two CARD domains responsible for downstream signalling to MAVS. Dicer possesses two RNase III domains involved in dsRNA dicing.
Figure from Maillard et al., EMBOJ, 2019
Publications
Key publications
Schierhorn KL, Sanchez‐David RY & Maillard PV (2022) Mammalian antiviral RNAi is on the move. Embo J: e111210
Sanchez-David, R.Y., and Maillard, P.V. (2021). Unlocking the therapeutic potential of antiviral RNAi. Immunity 54, 2180–2182.
Maillard PV, Van der Veen AG, Poirier EZ, Reis e Sousa, C. Slicing and dicing viruses: antiviral RNA interference in mammals. EMBO J. 2019;e100941
Van der Veen AG, Maillard PV, Marten Schmidt J, Lee SA, Deddouche-Grass S, Borg A., Kjaer S., Sniders AP, Reis e Sousa C. The RIG-I-like receptor LGP2 inhibits Dicer- dependent processing of long double-stranded RNA and blocks RNA interference in mammalian cells. EMBO J. 2018;37(4)
Maillard PV, Van der Veen AG, Deddouche-Grass S, Rogers NC, Merits A, Reis E Sousa C. Inactivation of the type I interferon pathway reveals long double-stranded RNA-mediated RNA interference in mammalian cells. EMBO J. 2016;35:2505-2518
Maillard PV, Ciaudo C, Marchais A, Li Y, Jay F, Ding SW, Voinnet O. Antiviral RNA interference in mammalian cells. Science. 2013;342:235-8
All publications
Supervision
Please visit the Maillard lab webpage for more information.
Current team
Postdoctoral researchers:
- Dr Josephine Nemegeer
- Dr Raul Y. Sanchez-David
PhD students:
- James Holt (co-supervised with Dr Helen Rowe, Centre for Immunobiology)
Research assistant:
Lab Alumni
- Dr Kristina Schierhorn, postdoctoral researcher from December 2020 to March 2023
- Giulia Andreea Ionescu, technician from July 2022 to January 2023
- Tamador Elamin, MSc student from March to July 2023
- Hafsa Duale: MSc student from March to July 2022
- Sarah Louise Long, MSc student from March to July 2021
- Tamara Lopez-Vidal. MSc student March to August 2019
- Tomáš Demeter, MSc student, internship funded by Erasmus from June to August 2017
If you are interested in joining our team, please contact Pierre Maillard (p.maillard@qmul.ac.uk).