Latest News and Events
Paper on AI/ML and ITP
We are pleased to announce the publication of our first paper on Artificial Intelligence (AI) & Machine Learning (ML) and its application in ITP, titled ‘Can Machine Learning Assist in Diagnosis of Primary Immune Thrombocytopenia? A Feasibility Study’. This was a joint collaboration with the School of Electronic Engineering & Computer Science, Queen Mary University of London.
We look forward to building on this work and further exploring the field of AI/ML and its application in healthcare.
The paper can be downloaded from https://www.mdpi.com/2075-4418/14/13/1352.
Substantial Amendment 8 - Change of Study CI
 We have submitted a substantial protocol amendment to notify the change of CI for the UK Adult ITP Registry Study. Dr Frederick Chen (right) has taken over the role of Chief Investigator (CI), form Dr Vickie McDonald. The REC favourable opinion and HRA/HCRW approvals were received on 9th October 2023. The substantial amendment pack with all the documents and approvals can be downloaded from the following link:
We have submitted a substantial protocol amendment to notify the change of CI for the UK Adult ITP Registry Study. Dr Frederick Chen (right) has taken over the role of Chief Investigator (CI), form Dr Vickie McDonald. The REC favourable opinion and HRA/HCRW approvals were received on 9th October 2023. The substantial amendment pack with all the documents and approvals can be downloaded from the following link:
Substantial amendment 8 pack for sites [10,348KB]
If you have any questions, please do not hesitate to contact us.
Blood sample collection
The ITP Registry team is now accepting blood samples from all sites again.
If there are any patients who have previously consented to donating bloods for the registry study but you have not taken any bloods due to the hold, please go ahead and obtain the bloods from the relevant patients when they next attend your hospitals/clinics.
Full details relating to the blood sample collection aspect of the protocol can be found in the following link.
Study recruitment extension
We have submitted a non-substantial amendment to extend the recruitment end date to 31st December 2025. An email was sent out to all sites with the submission confirmation and amendment pack; if you require this, please contact the registry team.
Updated database to capture COVID-19 data
The REDCap database for the UK Adult ITP Registry has been updated to include COVID-19 infections in ITP patients, and can be found under the comorbidities section of the REDCap database, as with other infections. This is a simple dataset capturing information about the severity of ITP. We will also capture vaccination.
Whilst updating the database, we have also added Fostamatinib and Avatrombopag as treatment options. A minor amendment has been processed and the data capture forms have been updated accordingly.
Do let us know if you have any questions around this.
Remote consenting - Updated study documents
We have recently updated our protocol and related study documents to enable sites to consent potential patients remotely, i.e. not requiring to see the patient in person in hospital/clinic. The amendment pack has been sent to all sites and can be implemented with immediate effect.
The latest study documents can be found on the following link.
Statement on COVID-19
Due to the COVID-19 outbreak in the UK, there is a possibility that sites may not be able to recruit patients into the registry. Patient safety is paramount and we understand if hospitals decide to halt recruitment for the UKITPR in line with their local policies and use clinical staff aligned to research projects to support NHS resource demands. You do not have to inform us that this is the case; please proceed as instructed by your local research management teams.
In addition to this, we are requesting for sites to not collect or send DNA samples for patients in the ITP registry until further notice. We will keep you posted with further developments as needed.
Thank you for your hard work.
Please do not hesitate to contact us if you have any questions or concerns.
UK Adult ITP Registry – Update January 2020
Moving into 2020, and after a very busy 2019, we are delighted to inform you that 2019 was the highest recruiting year on record for the UK Adult ITP registry with a total of 544 adults recruited. In addition to this, 19 patients have been recruited to the pregnancy registry.
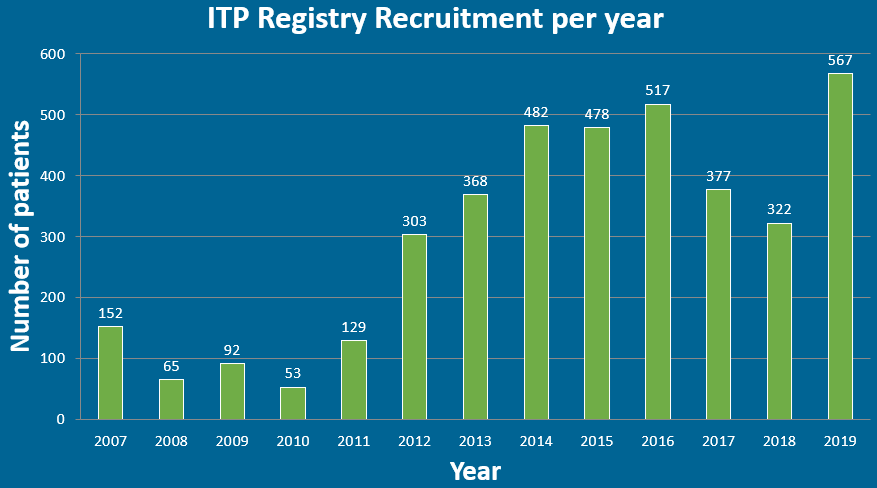
Ahead of the next newsletter, we wanted to share this news and say a big thank you to all the teams involved for the hard work. We look forward to further development of the registry in year ahead.
Wishing you all a very happy and productive 2020.
Recruitment activity on the Central Portfolio Managment System
You may already be aware that participant entry into the NIHR Central Portfolio Management System (CPMS) for trials is changing. As of 1st June 2019, sites are expected to upload their own recruitment figures on to a Local Portfolio Management System (LPMS). This can either be EDGE, ReDA or an alternative system, depending on whether your site is in England, Northern Ireland, Scotland or Wales.
This will have an impact on how recruitment to the registry is recorded. The new process will be as follows:
-
Sites consent the patient locally.
-
Sites upload their recruitment onto their LPMS. This then feeds through onto the CPMS.
-
Sites send their signed consent forms and updated participants logs to the UK ITP Registry inbox.
-
At the beginning of each month, the UK ITP Registry team will review the recruitment figures uploaded onto CPMS from the previous month and correlate with the participants logs received from each site to ensure there are no discrepancies.
-
Any discrepancies identified will be raised with the sites/NIHR as relevant.
-
Once resolved, UK ITP Registry team will confirm recruitment for that month.
If you haven’t sent through the consent forms and participant logs for all patients consented before 31st may 2019, please can you send these through as soon as possible, so that we can ensure that the recruitment figures on CPMS are correct up to the changeover.
If you have any queries relating to transition, please do not hesitate to contact us.