Rare disease differences in males and females: X Chromosome inactivation and reactivation – implications for a therapeutic approach
Rett syndrome is a rare and devastating neurological disorder. It is a genetic disease caused by a mutation on the X chromosome, and affects almost only girls. Andrea Cerase from the Centre for Genomics and Child Health discusses his research into rare disease differences between sexes, and the implications for treating epigenetics-based disorders.

Baby girls affected by Rett syndrome show impaired cognitive abilities and autistic behavior, often from their first year. This is because one of their X chromosomes has a mutation in the MECP2 gene. Baby boys, however, have one X chromosome and it almost never carries this mutation. Could this be an extreme case of “sexist mutation”, or have males found a way to protect their unique X chromosome?
Neither one nor the other actually: the mutation in the MECP2 gene on the X chromosome arises probably as often in female as in male fetuses. But female fetuses have a genetic advantage: since they have two X chromosomes, they only express the mutated one in about 50 per cent of their cells and still have about 50 per cent of healthy cells. The fetus doesn’t develop in an ideal way, but it can still live and the little girl is born with a severe disability. This is the consequence of a balancing process called ‘X chromosome inactivation’, by which one of the two X chromosomes in females is randomly silenced to achieve an equilibrium in X‐linked gene expression between sexes.
Male fetuses on the other hand have only one X chromosome which is then expressed in all cells: since nothing can compensate for the mutation, the fetus cannot develop properly and doesn’t survive birth. Very few boys are born with Rett syndrome because the fetuses affected never make it that far. Although it is counter-intuitive, it is actually the females who have a way to protect themselves from the MECP2 mutation on the X chromosome.
Understanding the mechanism underlying the silencing and/or awakening of the X chromosome could potentially lead to life-saving treatments
Because females silence about 50 per cent of the functional copy of any X-linked genes during the process of X inactivation, they are a living mosaic of cells expressing either the healthy (also called wild-type, WT) or mutated copy of a protein. As a consequence, cells expressing the mutated form of the protein may not work as well as the cells expressing the WT copy of this gene. Even in the cells expressing the mutated copy of a gene, they have a reservoir of the “dormant”, healthy copy of the gene that can be awakened (reactivated) in order to alleviate the disease. Indeed, the disease is often due to a reduced dose of functional protein which means it can no longer play its biological role.
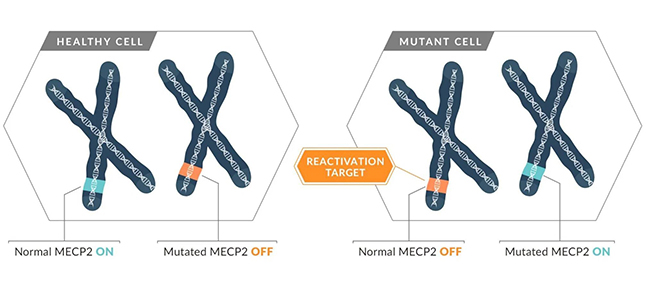
Credit: Rett Syndrome Research Trust – www.reverserett.org
The X chromosome is important for brain development
Many genes on the X chromosome regulate brain development and function. Reactivation of the inactive X chromosome can therefore be critically important for several genetic diseases, ranging from poorly-characterised genetic diseases such as CDKL5 and Fragile-X syndromes to more frequent and better-described diseases, such as Rett syndrome. These diseases are associated with autistic behavior or severe intellectual disability and neuropsychiatric traits.
From rare disorders to common diseases – the role of epigenetics
Compensation of the genetic differences between males and females is a reversible gene regulation mechanism, and it is also defined as an epigenetic mechanism (from Greek: on top of genetics). This type of reversible gene regulation is used by complex, multicellular organisms to generate different cells types during development, regulate cellular functions and to programme cell death and ageing. Therefore it is incredibly important to study these rare epigenetics-based diseases as the knowledge gained in these studies can be used for treating other genetic disorders, such as mental health disorders associated with epigenetic/reversible gene silencing such as depression, bipolar disorder, schizophrenia or some types of cancer.
More information
- Find out more about the Centre for Genomics and Child Health at the Blizard Institute.
- Find out more about Barts and The London School of Medicine and Dentistry, Queen Mary University of London.