| (CH3-CH2)4N+ | tetraethylammonium |
| Cl(CH3)3P+ | chlorotrimethylphosphonium |
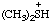 | dimethylsulfonium |
| (C6H5)2I+ | diphenyliodonium |
| Cl2F+ | dichlorofluoronium |
| CH3-C | ethylidyneoxonium |
Contents of this section
RC-82.0. Introduction. For the purposes of organic nomenclature, a cation is a molecular entity carrying at least one unit of positive charge formally derived from a parent hydride, a parent compound, or a hydro derivative of either, by the gain of one or more hydrons, by the loss of one or more hydride ions, or a combination of these operations. An atom at which a positive charge is considered to reside is called a cationic center. Cations with two or more cationic centers in the same structure are called dications, trications, etc.
Note. Hydron is a generic name for the hydrogen cation, i.e., the naturally occurring mixture of protons, deuterons, and tritons (ref 9). The name proton is restricted to the hydrogen cation having the mass number 1, i.e., 1H+.RC-82. 1. Cationic compounds with cationic centers derived formally by the addition of hydrons
Note. The procedure of RC-82.2.2.1 or RC-82.2.2.2 could be applied to skeletal atoms of parent hydrides in higher valence states that are described by the λ-convention (ref 16), but the method given here is preferred.RC-82.1.1. Cationic centers in parent hydrides
RC-82.1.1.1. Monocationic mononuclear parent cations. A parent cation derived formally by the addition of one hydron to a mononuclear parent hydride of the nitrogen, chalcogen, and halogen families (see RC-80.9.1) in its standard bonding number state (ref 16) is named by adding the suffix "-onium" to a root for the element as shown in Table 3.
Note. The term "carbonium" has long been used for a class of hydrocarbon cations derived formally by the loss of a hydride ion from a neutral hydrocarbon. More recently, the terms siliconium and even boronium have been used in an analogous manner. However, in organic nomenclature recommendations, these terms have not been used to name specific cations and they cannot now be used as a name for the cations H5C+ or H5Si+ by extending RC-82.1.1.1. These cations are to be named methanium and silanium, respectively, according to RC-82.1.1.2. Similarly, names such as carbenium and silenium have been used to describe classes of cations derived formally by addition of a hydron to the appropriate mononuclear divalent species (see RC-81.1.3.1). Except for the names carbenium, carbynium, and nitrenium, which may be used as alternatives to methylium (see RC-82.2.2.1), methyliumyl (see RC-85.2), and azanylium or aminylium (see RC-82.2.2.2), respectively, such names should not be used for naming specific compounds; they should be named according to RC-82.2.2.1 or RC-82.2.2.2, as appropriate. The general class name "carbocation" includes cationic hydrocarbons of both types. Modifying terms, such as tricoordinate and pentacoordinate, are added, if necessary, to differentiate between the two types.Table 3. Names for Mononuclear Parent Cations in IUPAC Systematic Substitutive Nomenclature
| H4N+ | ammonium* | H3O+ | oxonium | H2F+ | fluoronium |
| H4P+ | phosphonium | H3S+ | sulfonium | H2Cl+ | chloronium |
| H4As+ | arsonium | H3Se+ | selenonium | H2Br+ | bromonium |
| H4Sb+ | stibonium | H3Te+ | telluronium | H2I+ | iodonium |
| H4Bi+ | bismuthonium |
Examples:
| (CH3-CH2)4N+ | tetraethylammonium |
| Cl(CH3)3P+ | chlorotrimethylphosphonium |
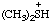 | dimethylsulfonium |
| (C6H5)2I+ | diphenyliodonium |
| Cl2F+ | dichlorofluoronium |
| CH3-C | ethylidyneoxonium |
Note 1: When a cationic center is fully substituted and locants are cited for the substituents, the locant for the cationic center is often considered unnecessary and omitted.Examples:Note 2: The operational suffix "-ium", when added to the name of a neutral parent hydride, indicates only that the number of substitutable hydrogen atoms at the position given by the appropriate locant has been increased by the addition of a hydron. Accordingly, there is no change in the bonding pattern at that position from that of the neutral parent hydride. Thus, specific structures formally derived by the addition of a hydron to one end of a double bond resulting in the generation of a tricoordinate carbocation at the other end of the double bond are named by RC-82.2. The procedure of Rule C-82.4 in the 1979 IUPAC Organic Rules (ref 2ee) is not included in these revised recommendations.
| H5C+ | methanium |
| [C2H7]+ | ethanium |
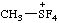 | tetrafluoro(methyl)-λ4-sulfanium (ref 16) |
Note The λn defines the neutral parent hydride SH4 (ref 16). The suffix "-ium" describes the addition of a hydron, H+, just as for a skeletal atom of a parent hydride in its standard valency state.
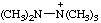 | pentamethyldiazanium pentamethylhydrazinium [traditional parent hydride name (ref 14)] |
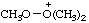 | trimethyldioxidanium |
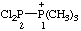 | 2,2-dichloro-1,1,1-trimethyldiphosphan-1-ium |
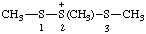 | 1,2,3-trimethyltrisulfan-2-ium |
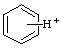 | benzenium |
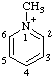 | 1-methylpyridin-1-ium |
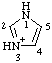 | 1H-imidazol-3-ium |
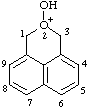 | 2-hydroxy-1H,3H-naphtho[1,8-cd]pyran-2-ium |
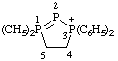 | 1,1,3,3-tetraphenyl-4,5-dihydro-3H-1λ5,2,3-triphosphol-3-ium |
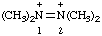 | tetramethyldiazene-1,2-diium |
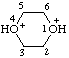 | 1,4-dioxane-1,4-diium |
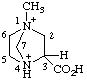 | 3-carboxy-1-methyl-1,4-diazabicyclo[2.2.1]heptane-1,4-diium 3-carboxy-1-methyl-1,4-diazoniabicyclo[2.2.1]heptane (see RC-82.4) |
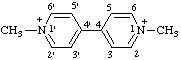 | 1,1'-dimethyl[4,4'-bipyridine]-1,1'-diium |
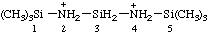 | 1,1,1,5,5,5-hexamethyltrisilazane-2,4-diium |

8,13,13-trimethyl-3,6,15,18-tetraoxa-8-thia-13-azaicosane-8,13-diium
8,13,13-trimethyl-3,6,15,18-tetraoxa-8-thionia-13-azoniaicosane (see RC-82.4)
RC-82.1.2. Cationic centers on characteristic groups. Although cationic centers derived formally by the addition of a hydron to a characteristic group can be named on the basis of parent cations derived from parent hydrides as given above in RC-82.1.1, it is often convenient and/or desirable to name a cation on the basis of a larger parent structure. Hence, the following recommendations for describing cationic centers located on some, but not all, of the characteristic groups of substitutive nomenclature are presented.
RC-82.1.2.1. Cationic suffixes. A cationic center formally derived by the addition of one hydron to a characteristic group listed in Table 4 is described by adding the appropriate cationic suffix given in Table 4, generated by adding the operational suffix "-ium" to the suffix name for the corresponding neutral suffix, to the name of an appropriate parent hydride.
Table 4: Suffixes for Cationic Characteristic Groups
| Characteristic Group Suffix | Cationic Group Suffix |
|---|---|
| -ic acid (oxo acids only) | -ic acidium |
| -amide | -amidium |
| -imide | -imidium |
| -nitrile | -nitrilium |
| -amine | -aminium |
| -imine | -iminium |
Note: Amides of carboximidic and sulfinimidic acids were named as amidines, carboxamidines, or sulfinamidines in the 1979 IUPAC Organic Rules (ref 2y); the corresponding suffixes for monocationic centers on these suffixes would be "-amidinium", "-carboxamidinium", and "-sulfinamidinium".When one of these characteristic group suffixes (except for "-ic acid") implies two or more of the same characteristic group, as, for example, in the name of a parent compound such as malonamide, the corresponding cationic group suffix denotes the addition of one hydron to each implied characteristic group.
Note: This method does not require knowledge, or assumption, of a specific structure, although the use of italicized element symbols, where unambiguous, can provide specificity. This is a definite advantage where it is not possible, or not desirable, to locate position(s) of the cationic center(s) on specific atoms of a characteristic group; this is particularly useful for unsubstituted cations derived from characteristic groups with two or more heteroatoms. This method also retains a correlation with the functionality of the corresponding neutral compound, and, in most cases, allows the use of a larger parent structure as the basis for the name. Names based on parent hydrides (RC-82.1.1) should be used if it is necessary or desired to indicate a specific structure.Examples:
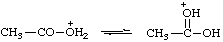 | acetic acidium |
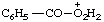 | peroxybenzoic OO-acidium perbenzoic OO-acidium |
Note: The locant symbol OO, although distinguishing between the carbonyl oxygen atom and the oxygen atoms of the peroxy group, does not distinguish between the oxygen atoms of the peroxy group.
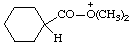 | O,O-dimethylcyclohexanecarboxylic acidium cyclohexanecarbonyldimethyloxonium (RC-82.1.1) |
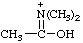 | N,N-dimethylacetimidic acidium (1-hydroxyethylidene)dimethylammonium (RC-82.1.1) |
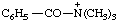 | N,N,N-trimethylbenzamidium |
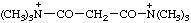 | N,N,N,N',N',N'-hexamethylmalonamidium |
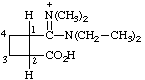 | 2-carboxy-N,N-diethyl-N',N'-dimethylcyclobutane-1-carboximidamidium (see footnote to Table 4 in RC-82.1.2.1) |
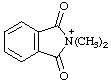 | N,N-dimethylphthalimidium 2,2-dimethyl-1,3-dioxo-2,3-dihydro-1H-isoindol-2-ium (RC-82.1.1.2) |
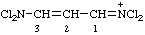 | N,N-dichloro-3-(dichloroamino)prop-2-en-1-iminium (ref 2z) dichloro[3-(dichloroamino)prop-2-en-1-ylidene]aminium (ref 2aa) |
Note: "Aminium" is used here in the same way as "aminyl"; see Note 2 and the third and fourth example under RC-81.2.3.1.
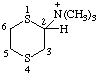 | N,N,N-trimethyl-1,4-dithian-2-aminium |
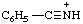 | benzonitrilium |
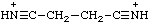 | succinonitrilium |
RC-82.1.2.2.1. Uronium ions and chalcogen analogs. Cations derived formally by the addition of a hydron to urea (or isourea) are named on the basis of the parent cation "uronium", representing the following tautomeric structures:
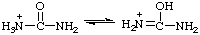
Locants follow those used for urea (ref 2ff) or isourea (ref 2gg). Chalcogen analogs are named on the basis of parent cations, such as "thiouronium", etc. When a choice between two or more tautomeric or mesomeric structures cannot be made the locants N, N', and O, S, etc., are used.
Examples:
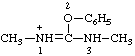 | 1,3-dimethyl-2-phenyluronium |
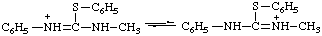
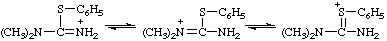
N,N-dimethyl-S-phenylthiouronium
RC-82.1.2.2.2. Cationic centers on implied characteristic groups other than uronium ions or chalcogen analogs. A cation other than a uronium ion, or a chalcogen analog, formally derived by the addition of one or more hydrons to a parent compound, the name of which implies a characteristic group from Table 4 in RC-81.1.2.1, is named by replacing the final "e" of the name of the compound, if present, by "-ium" or by adding "-ium", "-diium", etc., to the name of the parent compound.
Examples:
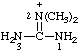 | 2,2-dimethylguanidinium |
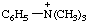 | N,N,N-trimethylanilinium |
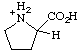 | prolinium |
Note: This method also may be used for cations covered by RC-82.1.2.1 and RC-81.1.2.2.Examples:
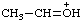 | ethylideneoxonium |
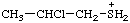 | (2-chloropropyl)sulfonium |
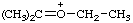 | ethyl(isopropylidene)oxonium (see RC-80.8) ethyl(propan-2-ylidene)oxonium (see RC-80.8) |
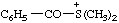 | benzoyldimethylsulfonium |
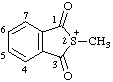 | 2-methyl-1,3-dioxo-1,3-dihydrobenzo[c]thiophen-2-ium 2-methyl-1,3-dioxo-1,3-dihydro-2-benzothiophen-2-ium (ref 3h) |
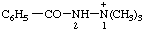 | 2-benzoyl-1,1,1-trimethyldiazan-1-ium 2-benzoyl-1,1,1-trimethylhydrazin-1-ium [traditional parent hydride name (ref 14)] |
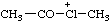 | acetyl(methyl)chloronium |
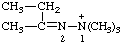 | 2-butan-2-ylidene-1,1,1-trimethyldiazan-1-ium (see RC-80.8) 1,1,1-trimethyl-2-(1-methylpropylidene)hydrazin-1-ium [traditional parent hydride name (ref 14)] (see RC-80.8) |
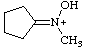 | cyclopentylidene(hydroxy)methylammonium |
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [y] Rules C-641.9, pp. 238-9; C-951.1, p. 292; C-951.2, p. 292; and C-951.3, p. 293; [z] Rule C-815.3(a), pp. 258-9; [aa] Rule C-815.3(b), pp. 258-9; [dd] Rule C-83.1, pp. 137-9; [ee] Rule C-82.4, p. 136-7; [ff] Rules C-971.1, p. 297; and C-971.2, pp. 297-8; [gg] Rule C-972.1, p. 298.
3. Reference 1, Section B, pp. 53-76: [h] Rule B3.5, pp. 67-8.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [c] Section I-8.4.2.2, p. 113.
9. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Physical Organic Chemistry, "Names for Hydrogen Atoms, Ions, or Groups and for Reactions Involving Them", Recommendations 1988, Pure Appl. Chem. 60, 1115-6 (1988).
14. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, "Nomenclature of Inorganic Chemistry: II.2. The Nomenclature of Hydrides of Nitrogen and Derived Cations, Anions, and Ligands (Recommmendations 1981)" Pure Appl. Chem. 54, 2545-52 (1982), Rule II.2.1, p. 2546.
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).