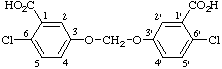
IUPAC name: 6,6'-dichloro-3,3'-(methylenedioxy)dibenzoic acid
CAS name: 3,3'-[methylenebis(oxy)]bis[6-chlorobenzoic acid]
Contents of this section
RC-80. Some general principles of IUPAC organic nomenclature used in these recommendations
Note. For convenience, modifications to the 1979 IUPAC Organic Rules (ref 1) to be included in the first part of a complete revision of the 1979 edition (ref 5) that are used in these recommendations are outlined here briefly.RC-80.1. Locants for suffixes indicating positions of operation or substitution in a parent hydride are to be placed preferably directly before the suffix to which they refer in uncontracted names, for example, but-2-ene, naphthalene-1,4-disulfonic acid, azulen-2-yl, and 9,10-butanoanthracen-2-yl. However, in contracted names such locants preferably precede the name, for example, 2-anthryl.
Note. In the 1979 IUPAC Organic Rules, locants are cited before such suffixes only when other locants appear in front of the name of the parent hydride, for example, 3H-indol-2-yl (ref 2d).RC-80.2. The subtractive suffixes "-ene", "-yne", etc., and hydro prefixes are used in the manner prescribed by the system in use for designating unsaturated or saturated positions in parent hydrides (ref 3b, 4b, 10a, 11a).
Note 1. Radical or ionic positions are preferred to positions of unsaturation expressed by the subtractive suffixes for assigning low locants, when there is a choice. This is consistent with principles in the 1979 IUPAC Organic Rules (ref 2e). However, the hierarchy of structural features of organic compounds for numbering preferences is under further study by the Commission and changes may be incorporated in future recommendations.RC-80.3. Indicated hydrogen is used in the manner given in the 1979 IUPAC Organic Rules for parent hydrides (ref 10b, 11b) and for principal characteristic groups and free valence positions ("added hydrogen") (ref 2g).Note 2. Although the 1979 IUPAC Organic Rules provide that hydro prefixes may be either detachable or nondetachable (ref 2f), in these recommendations and in the first part of a complete revision of the 1979 edition (ref 5) they are treated as nondetachable. Although at this time the Commission favors treatment of hydro prefixes as nondetachable, this principle has important implications for the numbering of organic structures and is therefore under continued study by the Commission.
Note. The concepts of indicated and "added" hydrogen are under study by the the Commission.RC-80.4. All neutral substituents of radical or ionic parent hydrides are expressed by the usual prefixes of organic nomenclature (ref 1, 5). In other words, suffixes expressing neutral characteristic groups are not added to names of radical or ionic parent hydrides. However, suffixes expressing ionic characteristic groups can be added to names of ionic parent hydrides (see RC-82.5.4, RC-83.4.4, and RC-84.1.2), and suffixes expressing neutral or ionic characteristic groups may be added to names of zwitterionic parent hydrides (see RC-84.1.1).
Note. The principle maintained by this recommendation is that the name of a radical should end with a radical suffix and the name of a nonradical ionic compound should end with an ionic suffix.RC-80.5. The operational suffixes "-ylene" (ref 10c), "-ylidene" (ref 3c, 10d), and "-ylidyne" (ref 10e) used in Sections A and B of the 1979 IUPAC Organic Rules (ref 1) are retained in these recommendations, but with restricted meanings. The suffix "-ylene" is used to describe the substituent groups -CH2- (methylene), -CH2-CH2- (ethylene), and -C6H4- (phenylene) only; methylene is used only when two bonds from the CH2 group are not attached to a single atom of a parent structure. The suffixes "-ylidene" and "-ylidyne" are used to describe groups in which two and three hydrogen atoms, respectively, have been removed from the same skeletal atom of a parent hydride and the resulting bonds are attached to a single atom of a parent structure, thereby describing the presence of a double or triple bond, respectively, between the substituting group and a parent structure or a parent substituent.
RC-80.6. Substituent acyl groups derived from organic acids are expressed by prefixes derived from the name of the acid, for example, cyclohexanecarbonyl (from cyclohexanecarboxylic acid), and phenylphosphonyl (from phenylphosphonic acid); however, this concept is still under study by the Commission.
Note. In the 1979 IUPAC Organic Rules, acyl groups as substituents are expressed by means of trivial names or by compound prefixes (ref 2h), for example, acetyl and cyclohexylcarbonyl.
RC-80.7. Sulfenic acid (ref 2i), selenenic acid (ref 2j), and related names, such as sulfenamide and sulfenyl, are not recommended in the first part of a complete revision of the 1979 edition of the IUPAC Organic Rules (ref 5), and therefore are not used in these recommendations.
RC-80.8. Substituent prefixes derived from acyclic parent hydrides. In these recommendations, a substituent prefix derived from an acyclic parent hydride named by the full systematic procedure, i.e., by replacement of the final "e" of the parent hydride name by suffixes such as "-yl" and "-ylidene" or by adding suffixes such as "-diylidene" and "-diyl" to the parent hydride name, may designate free valences at any position along an unbranched chain. This procedure is consistent with that given in Section D of the 1979 IUPAC Organic Rules (ref 4c, 10f) and supersedes the procedure given in Section A (ref 10g) of the 1979 IUPAC Organic Rules and the ACS recommendations for naming silanes (ref 12a), in which the principal chain must terminate at the free valence position. It must be noted that this procedure requires the citation of the locant "1" when the free valence position is at a terminal chain position, for example, propan-1-yl. However, use of "contracted" substituent prefix names, for example, propyl, still defines the free valence at a terminal position of the chain and no locant need be cited; thus, the names propyl and propan-1-yl are synonymous for CH3-CH2-CH2-, and the names isopropyl and propan-2-yl are synonymous for (CH3)2CH-.
The names for the substituent groups HS-, HSe-, and HTe- as prefixes are "sulfanyl-", "selanyl-", and "tellanyl-" in these recommendations, rather than "mercapto-" (ref 2k), "hydroseleno-" (ref 2j), [and presumably "hydrotelluro-" (ref 2m)], as in the 1979 IUPAC Organic Rules (ref 1).
RC-80.9. Systematic names for acyclic parent hydrides
RC-80.9.1. Names for mononuclear parent hydrides of twenty-one elements for use in systematic substitutive nomenclature are given in Table 2.
Note. Except for BiH3, CH4, and the chalcogen hydrides, these names are formed by adding "ne" to the replacement ("a") prefixes (ref 3d, 4d). Because of conflicts with Hantzsch-Widman names for heteromonocycles (ref 3e, 11), stems for mononuclear hydrides of bismuth and the chalcogen elements are "bismuth", "oxid", "sulf", "sel", "tell", and "pol".Table 2. Names for Mononuclear Parent Hydrides in IUPAC Systematic Substitutive Nomenclature
| BH3 | borane | NH3 | azane* | OH2 | oxidane | FH | fluorane |
| CH4 | methane | PH3 | phosphane | SH2 | sulfane | ClH | chlorane |
| SiH4 | silane | AsH3 | arsane | SeH2 | selane | BrH | bromane |
| GeH4 | germane | SbH3 | stibane | TeH2 | tellane | IH | iodane |
| SnH4 | stannane | BiH3 | bismuthane | PoH2 | polane | AtH | astatane |
| PbH4 | plumbane |
RC-80.9.2. Polynuclear hydrocarbons (ref 10h). The names ethane, propane, and butane for the unbranched saturated acyclic hydrocarbons C2H6, C3H8, and C4H10 are retained. The names for higher polynuclear unbranched saturated hydrocarbons are formed by combining a numerical prefix equal to the number of carbon atoms in the chain with the ending "ane", for example, pentane, icosane, and tritriacontane.
RC-80.9.3. Names for polynuclear acyclic parent hydrides other than hydrocarbons are formed by adding a numerical prefix equal to the number of atoms of the element in the chain to the name of the mononuclear hydride in RC-80.9.1 (ref 4e), for example, disilane, triazane, tetrasulfane.
RC-80.10. Radicofunctional nomenclature (ref 2p). Since this system of nomenclature is deemphasized in the first part of a revision of the 1979 edition of the IUPAC Organic Rules (ref 5), radicofunctional nomenclature, or that which might be considered as such in origin, for example, methylamine, will not be used for naming derived radicals or ions in these recommendations. However, the radicofunctional method for naming ions, i.e., the citation of the class name "cation" or "anion" as a separate word following the name of the radical, is included, but only as a less preferred alternative. Although useful for simple ions, its value for describing complex polyionic structures is severely reduced because prefix names for indicating ionic substituents cannot be generated.
RC-80.11. Multiplicative nomenclature. Two methods extensively used for naming assemblies of identical units that involve divalent or multivalent substituent prefix names will be used in these recommendations.
One method, codified in the 1979 IUPAC Organic Rules (ref 2q), multiplies only identical parent compounds, i.e., parent hydrides and a principal characteristic group, if any. This method requires that a connecting multipart divalent or polyvalent substituent prefix name be symmetrical. Substituents to the parent hydride, other than the principal characteristic group, are expressed by prefixes cited in front of the divalent or multivalent substituent prefix name.
The other method, used in CAS index nomenclature (ref 13a), multiplies two or more identical parent hydrides and all attached substituents, including principal characteristic groups, if any. In this method, all substituents attached to the parent hydride must be symmetrically positioned.
Note. Prior to 1972 (Volume 76), unsymmetrical two-part multiplying substituent prefix names were also permitted in CAS index nomenclature.Example:
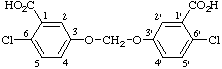
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [d] Preamble, Conventions (1), p. 80; [e] Subsection C-0.15, pp. 105-7; [f] Rule C-16.1, p. 108; [g] Rule C-315.1, p. 172; [h] Rule C-403.2, pp. 185, 188, Note, p. 188; and Rule C-631.1, pp. 230-1; [i] Rule C-521, p. 217; and Rule C-641.1, p. 233-4, Table XII, p. 234; [j] Rule C-701.1, p. 247-8, Table XIII; p. 248; [k] Rule C-502, pp. 210-1; [m] Rule C-701.2, p. 248; [n] Rule C-11.41, p. 91; [p] Subsection C-0.2, pp. 112-3; [q] Rules C-72, p. 130-1; and C-73, p. 132.
3. Reference 1, Section B, pp. 53-76: [b] Rule B-1.2 (in part). p. 54; [c] Rule B-5.12, pp. 70-1; [d] Rule B-1.1, pp. 53-5, Table I, p. 53; [e] Rule B1.1, pp. 53-5.
4. Reference 1, Section D, pp. 323-471: [b] Rules D-4.12, p. 374; D-4.13, p. 374; D-4.14, pp. 374-5; D-4.22, pp. 375-6; and D-4.23, p. 376; [c] Rule D-4.14, p. 374-5, esp. footnote, p. 374; and Rule D-6.12, pp. 409-10, Note. p. 410; [d] Appendix, Table I, pp. 459-60; [e] Rules D-4.11, p. 373; and D-6.11, p. 409.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
10. Reference 1, Section A, pp. 5-52: [a] Rules A-3.1, p. 11; A-3.2, p. 12; A-3.3, p. 12; A-11.3, p. 16; A-23.1, pp. 27-8; A-23.2, p. 28; A-31.3, p. 32; and A-41.3, p. 38; [b] Rule A-21.6, p. 25; [c] Rules A-4.2, p. 14; A-4.3, p. 15; A-11.6, pp. 17-8; A-24.4, p. 30; and A-55, p. 45-6; [d] Rules A-4.1, p. 14; A-4.4, p. 15; A-4.5, pp. 15-6; A-11.5, p. 17; and A-24.3, p. 30; [e] Rules A-4.1, p. 14; and A-4.4, p. 15; [f] Rule A-4, p. 14, ** footnote; [g] Rules A-1.2, p. 5; A-2.25, pp. 6-7; A-3.5, p. 13; A-4.1, p. 14; and A-4.4, p. 15; [h] Rule A-1.1, p. 5.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982, Pure Appl. Chem. 55, 409-16, 1983; [a] ibid. Recommendation RB-1.5; [b] ibid. Recommendation RB-1.2.
12. American Chemical Society, "Report of the ACS Nomenclature, Spelling and Pronunciation Committee for the First Half of 1952": [a] F. Organosilicon Compounds, Chem. Eng. News 30, 4517-22 (1952).
13. American Chemical Society, "Chemical Substance Index Names", Appendix IV in the 1987-1991 Chemical Abstracts Index Guide, Chemical Abstracts Service, Columbus, Ohio, 1992: [a] §125, p. 129I-130I.