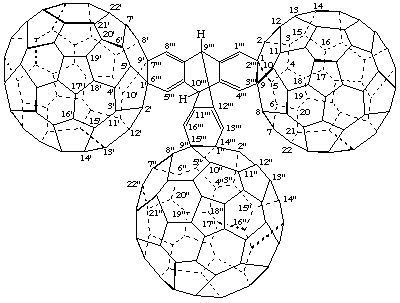
9'''H,10'''H-9,10-[1,2]Benzenoanthraceno[2''',3''':1,9;6''',7''':1',9';14''',15''':1",9"]tri(C60-Ih)[5,6]fullerene
[three (C60-Ih)[5,6]fullerenes joined by the bridged fused ring system 9,10-[1,2]benzenoanthracene]
Examples:
Example 1

9'''H,10'''H-9,10-[1,2]Benzenoanthraceno[2''',3''':1,9;6''',7''':1',9';14''',15''':1",9"]tri(C60-Ih)[5,6]fullerene
[three (C60-Ih)[5,6]fullerenes joined by the bridged fused ring system 9,10-[1,2]benzenoanthracene]
Example 2
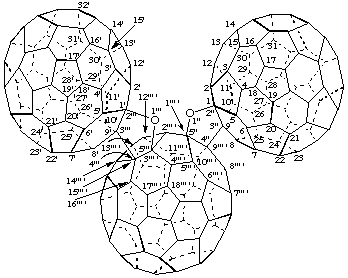
(C60-Ih)[5,6]Fullereno[9''' ',1''' ':4",5";15''' ',3''' ':4''',5''']difurano[2",3":1,9;2''',3''':1',9']di(C60-Ih)[5,6]fullerene)
[three (C60-Ih)[5,6]fullerenes connected by two furan rings; a fusion name based on the terminal fullerenes]
or
Bis[(C60-Ih)[5,6]fullereno[1',9':4,5]furano][2',3':1,9;2",3":3,15](C60-Ih)[5,6]fullerene
[three (C60-Ih)[5,6]fullerenes connected by two furan rings; a fusion name based on the central fullerene]
Example 3This tricomponent fullerene structure cannot be named by fusion principles. Nevertheless, there are three potential ways to name it.
1. Combine a fusion name for three (C60-Ih)[5,6]fullerenes joined by two fused cyclobutane rings plus the structure modifying prefix 'cyclo':
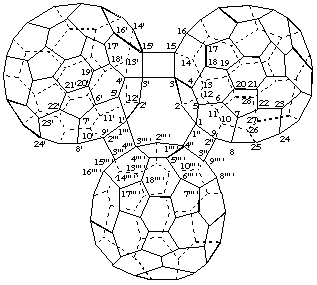
3,3':15,15'-Bis(cyclo)(C60-Ih)[5,6]fullereno[9''' ',1''' ':3",4";15''' ',3''' ':3''',4''']-
dicyclobuta[1",2":1,9;1''',2''':1',9']di(C60-Ih)[5,6]fullerene
[three (C60-Ih)[5,6]fullerenes connected by two cyclobutane rings; a fusion name based on terminal fullerenes plus two additional single bonds denoted by the structure-modifying prefix 'cyclo']
2. Cite one fullerene as a bridge between the other two fullerenes fused together by a cyclobutane ring.
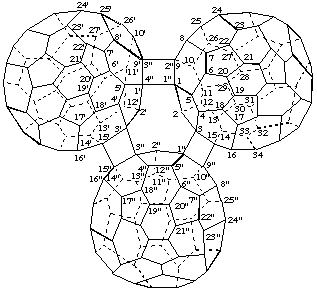
3,3',15,15'-([1,3,9,15]Epi(C60-Ih)[5,6]fullereno)cyclobuta[1",2":1,9;4",3":1',9']di(C60-Ih)[5,6]fullerene
[the bridging fullerene utilizes primed numbering, in this case double primes]
3. Adapt phane nomenclature (ref. 12) as follows:
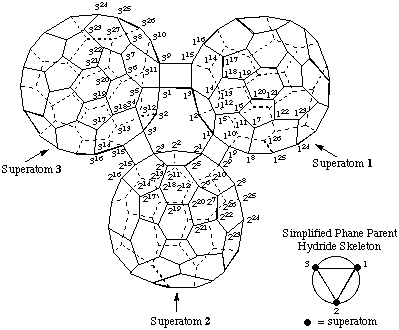
1,2,3(1,9,3,15)-Tri(C60-Ih)[5,6]fullerenatetracyclo[1.0.0.01,2.02,3]triphane
Example 4There are three ways to name this multicomponent fullerene consisting of four fullerenes interconnected by three cyclobutane rings.
1. Fused ring nomenclature.
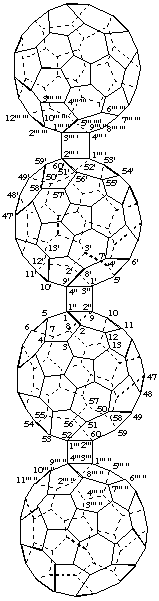
Bis[(C60-Ih)[5,6]fullereno[1',9':3,4]cyclobuta][1''',2''':52,60;1'''',2'''':52'.60']cyclobuta-
[1",2":1,9;3",4":1',9']di(C60-Ih)[5,6]fullerene
[two (C60-Ih)[5,6]fullerenocyclobuta components fused to a cyclobutadi(C60-Ih)[5,6]fullerene]
2. Ring assembly nomenclature plus 'cyclo'.
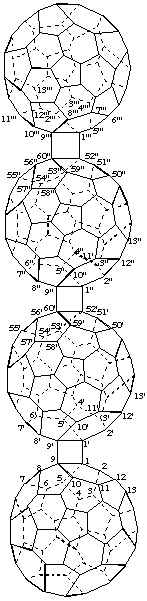
9,9':9",60':9''',60"-Tris(cyclo)-1,1':52',1":52",1'''-quater(C60-Ih)[5,6]fullerene
[a ring assembly name between four (C60-Ih)[5,6]fullerenes plus three additional single bonds denoted by the structure-modifying prefix 'cyclo']
3. An adaptation of phane nomenclature (ref. 12).
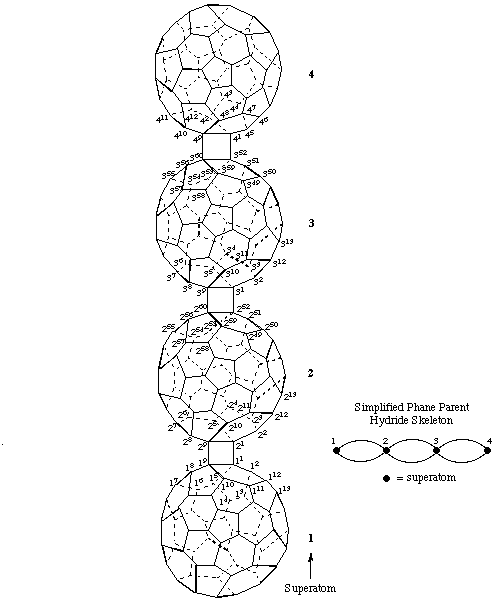
1,4(1,9),2,3(1,9,52,60)-Tetra(C60-Ih)[5,6]fullerenadispiro[1.0.13.02]tetraphane
Note: The spiro recommendations (ref 11) would not use superscript locants in the von Baeyer descriptor for a dispiro system. In this example these numbers are shown to clarify the unusual nature of the dispirotetraphane system.
11. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Extension and Revision of the Nomenclature for Spiro Compounds (IUPAC Recommendations 1999)". Pure Appl. Chem. 1999, 71, 531-558.
12. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Phane Nomenclature Part I: Phane Parent Names (IUPAC Recommendations 1998)". Pure Appl. Chem. 1998, 70, 1513-1545.