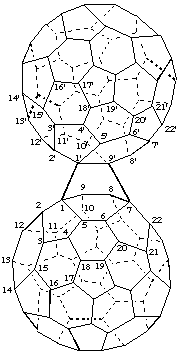
1,7-([1,9]Epi(C60-Ih)[5,6]fullereno)(C60-Ih)[5,6]fullerene
[a (C60-Ih)[5,6]fullerene bridging another (C60-Ih )[5,6]fullerene]
Contents of this section
Fu-10.1. Bridged and fused two-component fullerenes. One fullerene can be considered as bridging another fullerene, or be connected to another by a nonfullerene fusion component, or be part of a fusion component.
Examples:

1,7-([1,9]Epi(C60-Ih)[5,6]fullereno)(C60-Ih)[5,6]fullerene
[a (C60-Ih)[5,6]fullerene bridging another (C60-Ih )[5,6]fullerene]
Note: A bridging fullerene is not renumbered therefore its locants are primed.
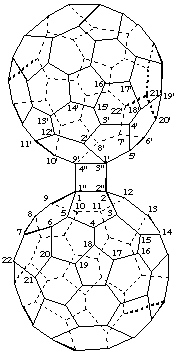
Cyclobuta[1",2":1,2;3",4":1',9']di(C60-Ih)[5,6]fullerene
[two (C60-Ih)[5,6]fullerenes fused to a cyclobutane ring]
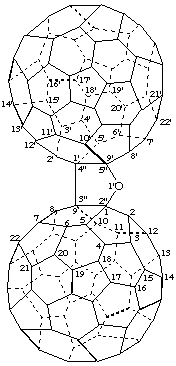
Furano[2",3":1,9;4",5":1',9']di(C60-Ih)[5,6]fullerene
[two (C60-Ih)[5,6]fullerenes fused to a furan ring]
Note: The CA index name is di[5,6]fullereno-C60-Ih-[1,9-b:1',9'-d]furan.
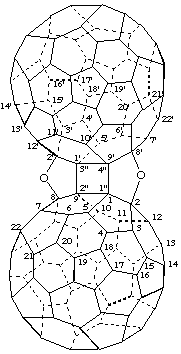
2,8':2',8-Diepoxycyclobuta[1",2":1,9;3",4":1',9']di(C60-Ih)[5,6]fullerene
[two bridges between two (C60-Ih)[5,6]fullerenes fused to a cyclobutane ring]
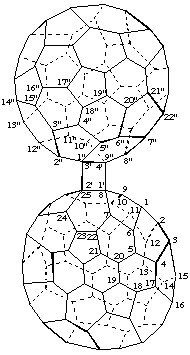
(C60-Ih)[5,6]Fullereno[1",9":3',4']cyclobuta[1',2':8,25](C70-D5h(6)]fullerene
[a (C60-Ih)[5,6]fullerene fused to a cyclobutane ring in turn fused to a (C70-D5h(6)]fullerene]
Note: Since a fused fullerene structure is not renumbered, each component must be identified by appropriate primed numbers.
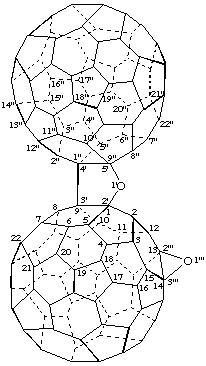
(C60-Ih)[5,6]Fullereno[1",9":4',5']furano[2',3':1,9]oxireno[13,14](C60-Ih)[5,6]fullerene
[an oxireno(C60-Ih)[5,6]fullerene and a (C60-Ih)[5,6]fullerene fused to a furan ring]
Examples:
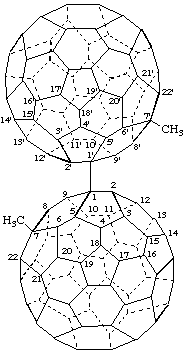
7,7'-Dimethyl-1,1'(7H,7'H)-bi(C60-Ih)[5,6]fullerene
[a ring assembly name; two (C60-Ih)[5,6]fullerenes linked by a single bond]
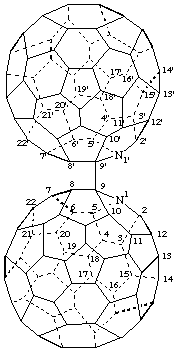
9,9'-Bi-1-aza(C60-Ih)[5,6]fullerene
[a ring assembly name; two 1-aza(C60-Ih)[5,6]fullerenes linked by a single bond; although the name of the azafullerene is 9H-1-aza(C60-Ih)[5,6]fullerene, the ring assembly does not require citation of indicated hydrogen]
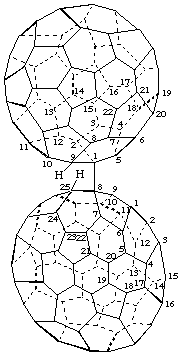
8-[(C60-Ih)[5,6]Fulleren-1(9H)-yl]-8,25-dihydro(C70-D5h(6))[5,6]fullerene
[ a (C70-D5h(6))fullerene substituted by a (C60-Ih)[5,6]fullerene]
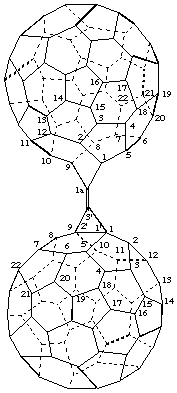
3'-[1aH-1(9)a-Homo(C60-Ih)[5,6]fulleren-1a-ylidene]-3'H-cyclopropa[1,9](C60-Ih)- [5,6]fullerene
[a cyclopropa(C60-Ih)[5,6]fullerene substituted by a homo(C60-Ih)[5,6]fullerene; the cyclopropa(C60-Ih)[5,6]fullerene is preferred on the basis of more rings]
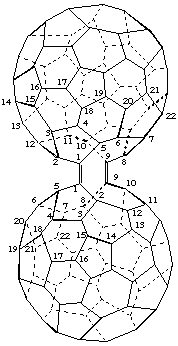
1,9-[1,9-Seco(C60-Ih)[5,6]fullerene-1,9-diylidene]-1,9-seco(C60-Ih)[5,6]fullerene
[a seco(C60-Ih )[5,6]fullerene substituted by an identical seco(C60-Ih)[5,6]fullerene or a seco(C60-Ih)[5,6]fullerene bridged by an identical seco(C60-Ih)[5,6]fullerene]
61,63,64,62-(1,9-Dinor(C60-Ih)[5,6]fullerene-2,5,8,10-tetrayl)-2,10:5,8-bis(ethene-1,2-diyl)-1,9-dinor(C60-Ih)[5,6]fullerene
[a bis(ethenediyl) bridged 1,9-dinor(C60-Ih)[5,6]fullerene substituted by a 1,9-dinor(C60-Ih)[5,6]fullerene]
or
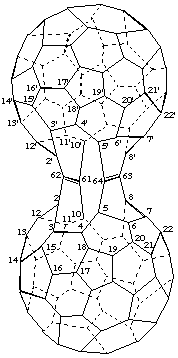
2,2',10,10':5,5',8,8'-Bis(ethene-1,1,2,2-tetrayl)bis[1,9-dinor(C60-Ih)[5,6]fullerene]
[two dinor(C60-Ih)[5,6]fullerenes bridged by two ethenetetrayl groups]
2,9,5-[1-Nor(C60-Ih)[5,6]fullerene-2,5,9-triyl)-1-nor(C60-Ih)[5,6]fullerene
[a 1-nor(C60-Ih)[5,6]fullerene substituted by a 1-nor(C60-Ih)[5,6]fullerene (numbering shown) or
a 1-nor(C60-Ih)[5,6]fullerene bridging a 1-nor(C60-Ih)[5,6]fullerene (the bridging component would have primed numbers)]
Examples:
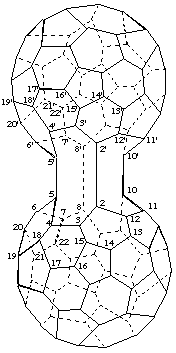
5,5':8,8':10,10'-Tris(cyclo)-2,2'-bi-1,9-dinor(C60-Ih)[5,6]fullerene
[a ring assembly name for two 1,9-dinor(C60-Ih)[5,6]fullerenes plus three additional single bonds designated by the structure-modifying prefix 'cyclo']
or
2,5,8,10-[1,9-Dinor(C60-Ih)[5,6]fullerene-2,5,8,10-tetrayl]-1,9-dinor(C60-Ih)[5,6]fullerene
[a 1,9-dinor(C60-Ih)-[5,6]fullerene substituted or bridged by another 1,9-dinor(C60-Ih)-[5,6]fullerene]
1H,1'H-25,25':26,26':45,45':46,46'-Tetrakis(cyclo)-24,24'-bi(C70-D5h(6))[5,6]fullerene
[a ring assembly name between two (C70-D5h(6))[5,6]fullerenes plus four additional single bonds designated by the structure-modifying prefix 'cyclo']
or
1H,1'H-Hexacyclo[4.4.0.02,5.03,9.04,8.07,10]decano[1",2",3",9",10":24,25,26,46,45;4",5",6",7",8":24',25',26',46',45']di-
(C70-D5h)[5,6]fullerene
[two (C70-D5h(6))[5,6]fullerenes joined by the von Baeyer ring system hexacyclo[4.4.0.02,5.03,9.04,8.07,10]decane]
Fu-10.4. Spiro homo and fused ring fullerenes are named by the established nomenclature for spiro ring systems containing at least one polycyclic ring system (ref. 11).
Examples:
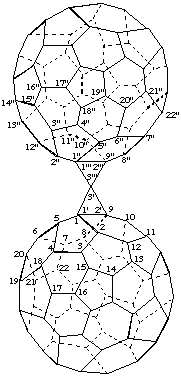
3',3'''-Spirobi[cyclopropa[1,9](C60-Ih)[5,6]fullerene]
[two cyclopropa(C60-Ih)[5,6]fullerenes linked by a spiro fusion]
Spiro[cyclopropa[1,9](C60-Ih)[5,6]fullerene-3',1"a-[1(9)a]homo(C60-Ih)[5,6]fullerene]
[a cyclopropa(C60-Ih)[5,6]fullerene spiro fused to a homo(C60-Ih)[5,6]fullerene]
8. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993: (a) Appendix, R-9.3, Table 33, p. 182; (b) R-2.4.4, Ring Assemblies, pp. 53-55; (c) R-0.2.4.2, Lowest set of locants, p. 17.
11. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Extension and Revision of the Nomenclature for Spiro Compounds (IUPAC Recommendations 1999)". Pure Appl. Chem. 1999, 71, 531-558.