SUBSoNIC
Full Title: Randomised double-blind efficacy and mechanism study of sub-sensory sacral (optimised) neuromodulation in adults with faecal incontinence 
Chief Investigator: Professor Charles Knowles
Funder: NIHR Efficacy and Mechanism Evaluation (EME) Programme/ Medtronic Inc
Funder Reference: 14/144/08
Research Status: Recruiting
Faecal incontinence is defined as the involuntary loss of stool or wind leading to a social or hygienic problem. It affects 1 in 10 people in the community, up to 3 in 10 people in the hospital, and up to 7 in 10 people in nursing homes. It can lead to negative effects on quality of life in terms of physical and emotional health. Treatment of faecal incontinence starts with drugs (usually loperamide), behavioural therapies and pelvic floor physiotherapy. When these therapies have failed, surgical treatment is considered as the next step.
A relatively new surgical treatment called sacral neuromodulation (SNM) is now commonly offered to adults suffering with faecal incontinence. Suitable patients include those with faecal incontinence caused by childbirth, surgery, and advancing age. A battery powered unit is implanted into the lower back in the region of the sacrum (tailbone). This is connected to a specially developed lead with electrodes which rest on the nerves in the lower spine (most commonly the third sacral nerve). This stimulator then continuously sends electrical impulses to the nerves and muscles that control the lower bowel (rectum and anus). The result may improve continence.
Previous studies have reported a great benefit of SNM in some patients. Unfortunately, other patients can have little or no response. We are still unsure about how SNM restores bowel control. And we still do not know with certainty how effective SNM really is. SNM costs on average £10,000 per patient just for the equipment and while a very safe therapy in comparison with other surgery, SNM still has some risks e.g. infection and unwanted side-effects. It is therefore vital that these questions are answered.
This research aims to establish how SNM works and how well SNM works. Patients who meet the current national criteria for SNM will be invited to participate in our trial. They will undergo detailed evaluation of their symptoms. Some will also undergo specialist tests before and after treatment. These will study their anal and rectal function as well as their corresponding brain activity (using a special test called magnetoencephalography).
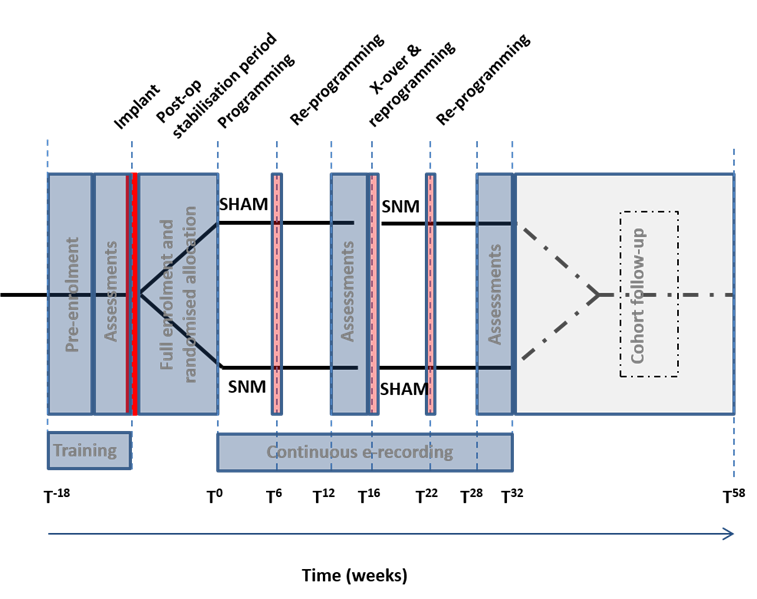
In contrast to a traditional clinical trial, the design of this trial will allow all patients to receive the treatment. This is possible by using a crossover design i.e. one where after implantation of the stimulator all patients receive a period of real therapy (SNM: device on but at an unperceived level of stimulation) compared to a period of sham (placebo) therapy. The effects of SNM can then be compared while both the patients and the research team are unaware whether the stimulation is SNM or sham. This is called ‘double-blinding’ and is the gold standard for determining what the true effects of the treatment are.
The study will be led by Barts Health NHS Trust and Queen Mary University of London. It will be performed in several NHS specialist centres and at the Aston Brain Centre (Birmingham). The trial will include a total of 90 patients. Each patient will participate for just over 12 months, but their SNM treatment will continue thereafter.
Background
Faecal incontinence is a substantial health problem, leading to substantive effects on quality of life in terms of physical and emotional health. Sacral neuromodulation (SNM) is considered the first-line surgical treatment option in adults with faecal incontinence in whom non-operative therapies have failed. However, the clinical efficacy of SNM has never been rigorously determined in a trial setting.
Design
Randomised double-blind crossover trial of sub-sensory sacral neuromodulation (SNM) and cohort follow up
Objectives
Primary Clinical Objectives:
- To determine the clinical efficacy of sub-sensory SNM compared to sham
Secondary Clinical Objectives:
- To obtain 1 year clinical outcomes of SNM using 2016 optimised therapy (with standardised lead placement)
- Validate a new electronic outcome measures and a device to record them
- To improve knowledge of the kinetics of effects of SNM
Mechanistic Objectives
- To identify the biological effect of sub-sensory SNM on underlying anorectal afferent neuronal pathophysiology
- To improve the general understanding of the pathophysiology of FI
Setting
NHS Trusts in U.K. and selected European sites with surgical expertise in SNM, and trial oversight by Pragmatic Clinical Trials Unit, Queen Mary, University of London
Inclusion Criteria
Adults aged between 18-75 meeting Rome III and ICI definitions of FI (recurrent involuntary loss of faecal material that is a social or hygienic problem and not a consequence of an acute diarrhoeal illness), have not responded to the NICE standard non-surgical treatments, patients with minimum severity criteria of 8 FI episodes in a 4 week screening period and clinically suitable for SNM.
Sample Size
N = 90 (1:1 allocation ratio)
Allocations
Group 1 (45): SNM/SHAM
Group 2 (45): SHAM/SNM
Methods
Suitably eligible patients consenting to take part in the study will undergo surgery to receive the SNM device (Medtronic Interstim II Model 3058) and be randomised to two equal groups (Group 1 and Group 2 above). Both groups will receive 16 weeks with SNM and 16 weeks with SHAM (in different order). At the beginning and part way through (+6 weeks) each phase, patients will attend for reprogramming of the SNM device. At the end of the 16 weeks they will cross over (SNM to SHAM or SHAM to SNM). Assessments will take the form of 4 week bowel diary and quality of life questionnaires / FI symptom scores. These will be completed at baseline (prior to SNM implantation & randomisation) and at the end of each 16 week phase.
After completion of both 16 week phases (SNM & SHAM), all patients will then be followed up to the 1 year time-point with all stimulators left SNM (open label cohort study) and patient decisive stimulation level (supra- or sub-sensory).
Analysis
Primary clinical outcome:
- Frequency of FI episodes per unit time using a paper diary (based on 4 weeks reporting)
Secondary clinical outcomes:
- Panel of validated FI clinical instruments/questionnaires
- Digital real-time event recording and novel outcomes
Mechanistic outcomes
- Advanced anorectal physiology
- Anocortical neurophysiology
Procedural data
- Electrode placement and settings
- Sensory and motor thresholds
Study Milestones:
Recruitment Start: 1 September 2017
Recruitment End: 28 February 2019
Complete Cross Over Assessments: 30 November 2019
Complete Cross Over Analysis: 30 March 2020
Results/Publication Cross Over Study: 31 May 2020
Results/Publication of Cohort Study: 30 November 2020
Additional Information
Please visit the public trial registry for more detailed information about this study as well as a link to the patient information sheet http://www.isrctn.com/ISRCTN98760715
Co-Applicants (Study Design Team)
|
Professor Knowles (Chief Investigator) Professor of Surgery Barts and the London School of Medicine and Dentistry Queen Mary University |
|---|
|
Reader in Medical Statistics Pragmatic Clinical Trials Unit Queen Mary University of London |
|
Consultant Colorectal Surgeon Southampton University Hospital NHS Foundation Trust |
|
Senior Lecturer in Gastroenterology University College Hospital London |
|
Professor of Clinical Neuroimaging Aston University Birmingham |
|
Professor in Medicine (Gastroenterology) University of Manchester |
|
Professor Of Anatomy University College Dublin |
|
Professor of Nursing King’s College London |
|
Professor of Surgery University College Dublin |
|
Senior Clinical Scientist Barts & the London School of Medicine and Dentistry Queen Mary University of London |
Research Team
|
Clinical Research Coordinator National Bowel Research Centre Queen Mary University of London 0207 882 8748 |
|---|
|
Clinical Trials Research Nurse National Bowel Research Centre Queen Mary University of London 0207 882 8751 |
|
Kerry Tubby Clinical Trials Coordinator National Bowel Research Centre Queen Mary University of London 0207 882 6032 |
|
Clinical Research Fellow National Bowel Research Centre Queen Mary University of London 0207 882 7882 |


