Extension and Revision of the von Baeyer System for Naming Polycyclic Compounds (Including Bicyclic Compounds)
(IUPAC Recommendations 1999)
VB-7
Continued from VB-6
Contents of Section
VB-7 Numbering of Secondary Bridges
References for this Section
VB-7 Numbering of Secondary Bridges
After numbering the main ring and main bridge (see VB-4) all independent secondary bridges are numbered before dependent secondary bridges. The numbering continues from the highest number of the main ring and bridge. Each secondary bridge is numbered in turn starting with the independent secondary bridge linked to the highest numbered bridgehead atom, then the independent secondary bridge linked to the next highest numbered bridgehead atom and so on. Each atom of a secondary bridge is numbered starting from the atom next to the higher numbered bridgehead.
NoteThis rule is based on the procedure used by CAS, which is consistent with the method used for the numbering of bridges across fused ring systems (see FR-8.6 and FR-8.7, ref 12). The previous version of the rule, A-32.23 (ref 6), has been interpreted in different ways. It is not clear if decreasing order refers to length of bridges or decreasing order of bridgehead locants.
Examples
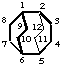
tricyclo[4.2.2.22,5]dodecane
(the secondary bridge is numbered starting from bridgehead 5)

tetracyclo[4.4.2.22,5.27,10]hexadecane
(the first secondary bridge to be numbered is linked to bridgehead 10)
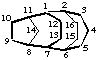
tetracyclo[5.4.2.22,6.18,11]hexadecane
(the first secondary bridge to be numbered is linked to bridgehead 11)
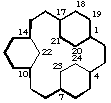
tetracyclo[15.2.2.24,7.110,14]tetracosane
(the first secondary bridge to be numbered is linked to bridgehead 14)
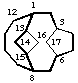
tetracyclo[6.4.3.13,6.113,15]heptadecane
(the first secondary bridge to be numbered is linked to bridgehead 15)
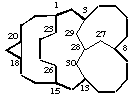
pentacyclo[13.7.4.33,8.018,20.113,28]triacontane
(the independent bridge is numbered before the dependent bridge)
VB-7.1 If there is still a choice lower locants are used for the atoms in the bridge linked to the higher numbered bridgehead.
Examples
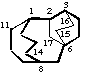
tetracyclo[6.3.3.23,6.12,6]heptadecane
(15 and 16 are the bridge to 3 not 2)
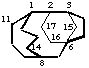
tetracyclo[6.3.3.22,6.13,6]heptadecane
(15 is the bridge to 3 not 2)
VB-7.2 If there is still a choice longer bridges are numbered before shorter bridges.
Example
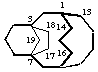
tetracyclo[7.4.3.23,7.13,7]nonadecane
References for this Section
6. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition, Pergamon Press, Oxford, 1979.
12. IUPAC Commission on the Nomenclature of Organic Chemistry, Nomenclature of fused and bridged fused ring systems (IUPAC recommendations 1998), Pure Appl. Chem., 70, 143-216 (1998).
Continued with VB-8
Return to von Baeyer Nomenclature home page.