Extension and Revision of the Nomenclature for Spiro Compounds
(IUPAC Recommendations 1999)
SP-8 to SP-9
Continued from SP-6 and SP-7
Contents of Section
SP-8 Ionic Spiro Atom
SP-9 Stereochemistry
References
SP-8 Ionic Spiro Atom
The preferred method for naming spiro compounds with an ionic spiro atom is to indicate a non-standard valency for the spiro atom and generate the ion by the appropriate subtractive or additive suffix (ref 7). Alternative methods use the ionic replacement terms (see R-5.8.2 and R-5.8.3, ref 6) or indicate the ion in the names of the components of the spiro system.
Examples:
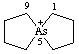
5λ5-arsaspiro[4.4]nonan-5-ylium (preferred name)
Alternative name 5-arsoniaspiro[4.4]nonane
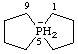
5λ5-phosphaspiro[4.4]nonan-5-uide (preferred name)
Alternative name 5λ5-phosphanuidaspiro[4.4]nonane
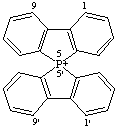
5λ5,5'-spirobi[benzo[b]phosphindol]-5-ylium (preferred name)
Alternative names 9-phosphonia-9,9'-spirobi[fluorene]
or 5,5'-spirobi[benzo[b]phosphindolium]
CAS index name 5,5'-spirobi[5H-dibenzophospholinium]
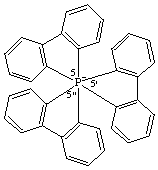
5λ7,5',5"-spiroter[benzo[b]phosphindol]-5-ide
The parent of this ion was called by CAS 5,5',5"-dispirotri[5H-dibenzophosphole] but is now named as an octahedral complex, tris([1,1'-biphenyl]-2,2'-diyl)phosphate(1-)
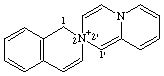
1H-2λ5-spiro[isoquinoline-2,2'-pyrido[1,2-a]pyazin]-2-ylium
CAS index name spiro[isoquinoline-2(1H),2'-[2H]pyrido[1,2-a]pyrazinium]
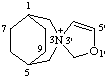
2'H-3λ5-spiro[3-azabicyclo[3.2.2]nonane-3,3'-[1,3]oxazol]-3-ylium
CAS index name spiro[3-azabicyclo[3.2.2]nonane-3,3'(2H)-oxazolium]
Note 2'H could be omitted.
SP-9 Stereochemistry
SP-9.1 Stereochemistry associated with tetrahedral geometry is indicated by the method of section E (ref 5) and R-7 (ref 6) (see also SP-1.8.10). Although most cases are straightforward there are some where Section E is inadequate. (See ref 17 for further details.)
Examples:
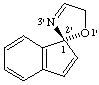
(R)-5'H-spiro[indene-1,2'-[1,3]oxazole]
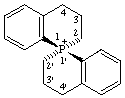
(S)-3,3',4,4'-tetrahydro-2H,2'H-1λ5,1'-pirobi[phosphinolin]-1-ylium (preferred name)
Alternative name (S)-3,3',4,4'-tetrahydro-2H,2'H-1-phosphonia-1,1'-spirobi[naphthalene]
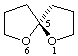
(S)-1,6-dioxaspiro[4.4]nonane
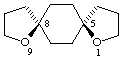
cis-1,9-dioxadispiro[4.2.4.2]tetradecane

cis-1,8-dioxadispiro[4.1.4.2]tridecane
or (5R,7S)-1,8-dioxadispiro[4.1.4.2]tridecane
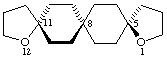
(5(11)Ra)-1,12-dioxatrispiro[4.2.2.411.28.25]nonadecane
Note The stereochemistry is indicated with a compound locant to indicate the chirality axis.
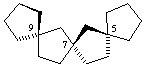
(R)-trispiro[4.1.1.49.27.25]heptadecane
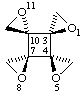
1r,5c,8c,11t-tetraoxatetraspiro[2.0.24.0.7.0.210.03]dodecane
Note Rules E-2.3.3 (ref 5) and R-7.1.1 (ref 6) would suggest r-1,c-5,c-8,t-11-tetraoxa.... but the Beilstein practice (ref 18) is used here as it is shorter and clearer. This change is being considered for the revision of section E (stereochemistry).
SP-9.2 Stereochemistry associated with non-tetrahedral geometry is indicated by the method of coordination chemistry (see I-2.2.3.2, I-10.5 and I-10.7, ref 19).
Examples:
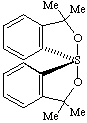
(TBPY-4-11'-A)-3,3,3',3'-tetramethyl-3H,3'H-1λ4,1'-spirobi[[2,1]benzoxathiole]
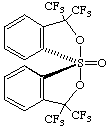
(TBPY-5-11'-A)-1-oxo-3,3,3',3'-tetrakis(trifluoromethyl)-3H,3'H-1λ6,1'-spirobi[[2,1]benzoxathiole]
or (TBPY-5-11'-A)-3,3,3',3'-tetrakis(trifluoromethyl)-3H,3'H-1λ4,1'-spirobi[[2,1]benzoxathiole] 1-oxide

(TBPY-5-12-C)-2-phenoxy-2λ5-spiro[1,3,2-dithiaphosphinane-2,2'-phenanthro[9,10-d][1,3,2]dithiaphosphole] 
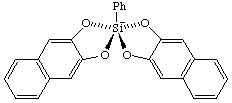
(SPY-5-21)-2-phenyl-2,2'-spirobi[naphtho[2,3-d][1,3,2]dioxasilol]-2-uide
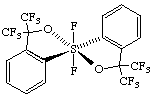
(OC-6-12)-1,1-difluoro-3,3,3',3'-tetrakis(trifluoromethyl)-3H,3'H-1λ6,1'-spirobi[[2,1]benzoxathiole]
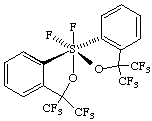
(OC-6-22-A)-1,1-difluoro-3,3,3',3'-tetrakis(trifluoromethyl)-3H,3'H-1λ6,1'-spirobi[[2,1]benzoxathiole] 
References
1. A. Baeyer, Systematik und Nomenclatur Bicyclischer Kohlenwasserstoffe, Ber. Dtsch. Chem. Ges. 33, 3771-3775 (1900). [Although he always published his papers as just Baeyer he was always referred to with the honorific as von Baeyer.]
2. D. Radulescu, Über die Nomenklatur der Spirane, Ber. Dtsch. Chem. Ges. 44, 1023-1026 (1911).
3. A.M. Patterson, Proposed international rules for numbering organic ring systems, J. Am. Chem. Soc. 47, 543-561 (1925); A.M. Patterson, The nomenclature of parent ring systems, J. Am. Chem. Soc. 50, 3074-3087 (1928); A.M. Patterson and L.T. Capell, The Ring Index, Reinhold, New York, 1940.
4. R.M. Beesley, C.K. Ingold and J.F. Thorpe, The formation and stability of spiro-compounds. Part I. spiro-Compounds from cyclo-hexane. J. Chem. Soc. 1080-1106 (1915) [This is the earliest paper found using the system but it makes no comment on this nomenclature]
5. IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition, Pergamon Press, Oxford, 1979.
6. IUPAC, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
7. IUPAC, Revised nomenclature for radicals, ions, radical ions and related species, recommendations 1993, Pure Appl. Chem. 65, 1357-1455 (1993).
8. Chemical Abstracts Service, Index Guide, 1997 appendix IV.
9. Based on a proposal by Andrey Yerin, ACD, Moscow.
10. J.E. Rush and L.J. White, Resolution of ambiguities in the nomenclature of spiro ring systems, J. Chem. Doc. 10, 195-204 (1970).
11. IUPAC, Treatment of variable valence in organic nomenclature (Lambda convention), recommendations 1983, Pure Appl. Chem. 56, 769-778 (1984).
12. IUPAC, Revision of the extended Hantzsch-Widman system of nomenclature for heteromonocycles (recommendations 1982), Pure Appl. Chem. 55, 409-416 (1983).
13. IUPAC, Glossary of class names of organic compounds and reactive intermediates based on structure, recommendations 1995, Pure Appl. Chem. 67, 1307-1375 (1995).
14. IUPAC-IUB, Nomenclature of steroids, recommendations 1989, Eur. J. Biochem. 186, 429-458 (1989); Pure Appl. Chem. 61, 1783-1822 (1989) and in Dictionary of Steroids (eds R.A. Hill, D.N. Kirk, H.L.J. Makin and G.M. Murphy) Chapman and Hall, London, 1991, pp xxx-lix; IUBMB, Biochemical Nomenclature and Related Documents, second edition, Portland Press, London, 1992, pp. 192-221.
15. IUPAC-IUBMB, Nomenclature of carbohydrates, recommendations 1996, Adv. Carbohydr. Chem. Biochem. 52, 43-177 (1997); Carbohydr. Res. 297, 1-90 (1997); J. Carbohydr. Chem. 16, 1191-1280 (1997); Pure Appl. Chem. 68, 1919-2008 (1996).
16. IUPAC, Nomenclature of fused and bridged fused ring systems, recommendations 1998, Pure Appl. Chem. 70, 143-216 (1998).
17. V. Prelog and G. Helmchen, Basic principles of the CIP-system and proposals for a revision, Angew. Chem., Int. Ed. Engl. 21, 567-583 (1982).
18. Stereochemical descriptors in Beilstein Handbook of Organic Chemistry, Fourth edition, Fifth Supplement, Series Vol. 18, Part 1, 1986, pp. XLVII-LXI.
19. IUPAC, Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific Publications, Oxford, 1990.
Return to Spiro home page.