Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999)
RF-7 and RF-8
Continued from RF-6. Fusion of Additional Rings
Contents of this section
- RF-7. Bridged Fundamental Parent Structures
- RF-8. Bond Order Modifications
- 8.1 Unsaturation
- 8.2 Saturation of Double Bonds
- 8.3 Introduction of Unsaturation
- 8.4 Rearrangement of a Double Bond
RF-7. Bridged fundamental parent structures.
Atomic bridges added to fundamental parent structures of natural products may be described by the methods used in systematic organic nomenclature [A-34 (ref 2), B-15.1 (ref 2) and R-9.2 (ref 3)]. This method is often used with hetero atom bridges. In fact, this method is often more useful than fusion procedures for describing certain types of heterocyclic rings fused to a fundamental parent structure, for instance, oxireno (epoxy) and thiireno (epithio). Bridge prefixes are always nondetachable.
Examples:
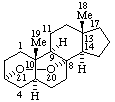 | 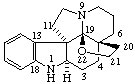 |
| 3α,8-Epidioxy-5α,8α-androstane | 19,21-Epoxyaspidospermidine |
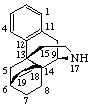 | 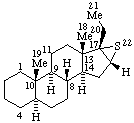 |
| 6β,14-Ethenomorphinan (may also be named 6α,14-Ethano-14α-morphin-7-ene) | 16α,17-Epithio-5α-pregnane (may also be named (16βH)-Thiireno[16,17]-5α-pregnane by RF-6)  |
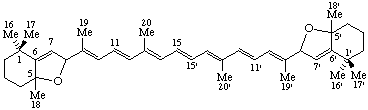
5,8:5',8'-Diepoxy-5,8,5',8'-tetrahydro-&beta,β-carotene
(note that this name follows the published recommendations for carotenoids (ref 5), in which the bridge prefix 'epoxy' is treated as detachable, and unprimed locants precede primed locants for 'hydro') 
RF-8. Bond Order Modification
RF-8.1. Unsaturation
Unsaturation in a compound whose parent structure (see RF-3 through RF-7) is fully saturated, or in the portion of a parent structure that is otherwise fully saturated, is indicated by changing -an to -ene, -adiene, -yne, etc.; -ane to -ene, -adiene, -yne, etc.; -anine to -enine, -adienine, -ynine, etc.; or -an- to -en-, -adien-, -yn-, etc. [see 3S-2.5 in the steroid nomenclature recommendations (ref 4), and Rule R-3.1.1 (ref 3)]. 
Examples:
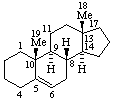 | 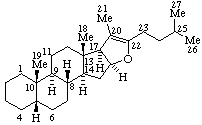 |
| Androst-5-ene | 5β-Furost-20(22)-ene |
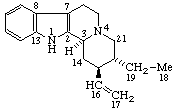 | 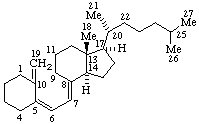 |
| Coryn-16-ene | (5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene |
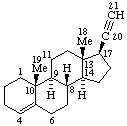 | 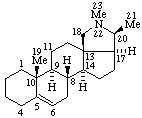 |
| Pregn-4-en-20-yne | Con-5-enine |
RF-8.2. Saturation of Double Bonds
Saturation of double bonds in a parent structure (see RF-3 through RF-7) whose name implies the presence of isolated double bonds and/or a system of conjugated double bonds is described by the prefix "hydro-", itself prefixed by an appropriate numerical term.
Examples:
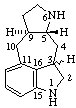
(3&zi;,9αH)-2,3-Dihydro-5(10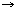 9)-abeoergoline
9)-abeoergoline 
7',8'-Dihydro-ε,γ-carotene
RF-8.3. Introduction of Unsaturation
The introduction of unsaturation additional to any implied in a parent structure (see RF-3 through RF-7) whose name does not end in "an", "ane" or "anine"; the conversion of an implied double bond to a triple bond; and the introduction of an additional double bond with rearrangement of an implied double bond, are denoted by the prefix "dehydro-", itself prefixed by a numerical term equal to the number of hydrogen atoms removed and the appropriate locants.
Examples:
9,10-Didehydroergoline
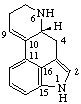
7,8-Didehydro-ε,ε-carotene
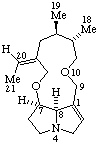 | 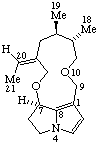 |
Senecionan
(fundamental parent structure) | 3,8-Didehydrosenecionan |
RF-8.4. Rearrangement of a Double Bond
Rearrangement of a double bond may be indicated by a combination of hydro and dehydro prefixes.
Examples:
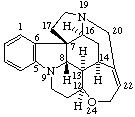 | 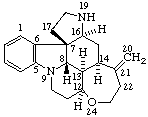 |
Strychnidine
(fundamental parent structure) | 20,21-didehydro-21,22-dihydro-19,20-secostrychnidine |
References for this section
2. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, l979 edition, Pergamon Press, Oxford, 1979.
3. International Union of Pure and Applied Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford, 1993. [Corrections see Pure Appl. Chem., 71, 1327-1330 (1999).]
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids", Pure Appl. Chem. 61, 1783-1822 (1989). [also in: Eur. J. Biochem. 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].
5. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "Nomenclature of Carotenoids", Pure Appl. Chem., 41, 405-431 (1975).
Continue with RF-9. Derivatives of Parent Structures
Return to Section F: Natural Products home page.
Return to IUPAC chemical nomenclature home page.


















