Phane Nomenclature, Part II
PhII-4
Substitutive nomenclature for phane parent hydrides
Continued from PhII-1 to PhII-3
Contents of this section
- PhII-4. Substitutive nomenclature for phane parent hydrides
- PhII-4.1. Phane parent hydrides with characteristic group suffixes.
- PhII-4.2. Radicals and ions derived from phane parent hydrides.
- PhII-4.3. Substituents of phane parent hydrides cited as prefixes
- References for this section
Continued with PhII-5 Phane parent hydrides modified by the addition or subtraction of hydrogen atoms
PhII-4. Substitutive nomenclature for phane parent hydrides
PhII-4.1. Phane parent hydrides with characteristic group suffixes.
In accordance with the fixed numbering of a phane parent hydride or phane parent hydrides modified by skeletal replacement ('a') nomenclature, characteristic groups cited as suffixes receive lowest possible locants (see PhII-1.1).
Examples:
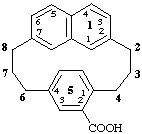
1(2,7)-naphthalena-5(1,4)-benzenacyclooctaphane-52-carboxylic acid
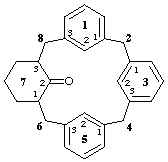
1,3,5(1,3)tribenzena-7(1,3)-cyclohexanacyclooctaphan-72-one

1,3,5,7(1,3)-tetrabenzenacyclooctaphane-12,32,52,72-tetrol
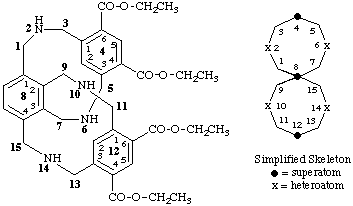
tetraethyl 2,6,10,14-tetraaza-4,12(1,3),8(1,3,2,4)-tribenzenaspiro[7.7]pentadecaphane-44,46,124,126-tetracarboxylate
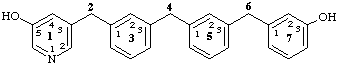
1(3)-pyridina-3,5(1,3),7(1)-tribenzenaheptaphane-15,73-diol
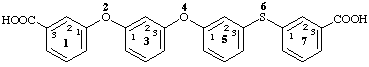
2,4-dioxa-6-thia-1,7(1),3,5(1,3)-tetrabenzenaheptaphane-13,73-dicarboxylic acid
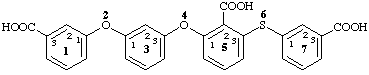
2,4-dioxa-6-thia-1,7(1),3,5(1,3)-tetrabenzenaheptaphane-13,52,73-tricarboxylic acid
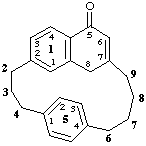
1(2,7)-naphthalena-5(1,4)-benzenacyclononaphane-15(18H)-one
(added hydrogen method)
or
15,18-dihydro-1(2,7)-naphthalena-5(1,4)-benzenacyclononaphan-15-one
(nondetachable 'hydro-' prefix method)
PhII-4.2. Radicals and ions derived from phane parent hydrides.
Radicals are named in the same way as substituent groups (see PhII-3). Ions may be named by use of ionic suffixes or by using ionic replacement ('a') prefixes; ionic suffixes, such as '-ylium', are preferred over the use of skeletal replacement ('a') prefixes (ref. 4a).
Example:
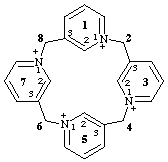
11λ5,31λ5,51λ5,71λ5-1(1,3),3,5,7(3,1)-tetrapyridinacyclooctaphane-11,31,51,71-tetrakis(ylium)
(preferred)
or
11,33,53,73-tetraazonia-1,3,5,7(1,3)-tetrabenzenacyclooctaphane
(numbering for this skeletal replacement name is based on the phane parent hydrocarbon)
PhII-4.3. Substituents of phane parent hydrides cited as prefixes
PhII-4.3.1.
Atoms and/or groups cited as detachable alphabetized prefixes are assigned positions according to the fixed numbering of phane parent hydride or phane parent hydrides modified by skeletal replacement ('a') nomenclature (ref. 3j).
Examples:
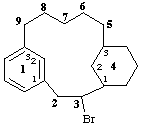
3-bromo-1(1,3)-benzena-4(1,3)-cyclohexanacyclononaphane
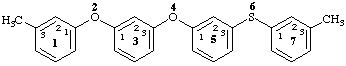
13,73-dimethyl-2,4-dioxa-6-thia-1,7(1),3,5(1,3)-tetrabenzenaheptaphane
PhII-4.3.2.
When, after application of PhII-4.3.1, a choice for low locants is necessary, the following criteria are considered in order until a decision can be made.
a. Low locants are assigned to the prefixes considered together as a set in ascending numerical order.
Example:
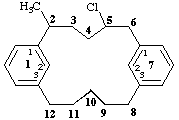
5-chloro-2-methyl-1,7(1,3)-dibenzenacyclododecaphane
b. Low locants are allocated in the order of citation in the name.
Example:
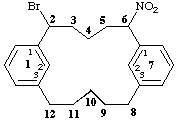
2-bromo-6-nitro-1,7(1,3)-dibenzenacyclododecaphane
References for this section
3. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry. A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, R. Panico, W. H. Powell and J.-C. Richer, eds. Blackwell Scientific Publications, Oxford, 1993. (j) R-0.1.8.3, pp. 10-12. (See also http://www.acdlabs.com/iupac/nomenclature/).
4. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry. "Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species (IUPAC Recommendations 1993)" Pure Appl. Chem. 65, 1357-1455 (1993). (a) RC-82.4, pp. 1411-1413. (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/ions/).
Continue with PhII-5 Phane parent hydrides modified by the addition or subtraction of hydrogen atoms
Return to Phane nomenclature part II home page
Return to IUPAC nomenclature home page












