IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
https://iupac.qmul.ac.uk/misc/ret.html
World Wide Web version prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
e-mail g.p.moss@qmul.ac.uk
These recommendations are as close as possible to the printed version prepared for publication by G.P. Moss [see Arch. Biochem. Biophys., 1983, 224, 728-731; Eur. J. Biochem., 1982, 129, 1-5; J. Biol. Chem., 1983, 258, 5329-5333; Pure Appl. Chem., 1983, 55, 721-726; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 247-251; copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. In the web version footnotes have been converted into notes following the paragraph to which they apply. In setting up the World Wide Web version an error was detected and the appropriate correction has been made. The change has been marked by which is a link to details of the change and where it applies. A PDF of the printed version is available.
Any comments should be sent to any member of the Committee
Contents
Funk [ref 1] suggested in 1912 the term 'vitamine' to describe a growth factor present in food which was essential for life. It later became clear that there was more than one growth factor, and McCollum [ref 2] divided them into two classes 'fat-soluble A' and 'water-soluble B'. Objecting to the chemical implications of the suffix '-ine', Drummond [ref 3] suggested deletion of the final 'e', renamed McCollum's two groups vitamin A and vitamin B, and proposed that further members of this series be called vitamin C, vitamin D, etc.
In 1934 Wald [ref 4] isolated from animal retina a substance he called retinene. Morton [ref 5] suggested that this compound was the aldehyde of vitamin A, and [ref 6] called it retinaldehyde (rather than retinal). The correct structure of vitamin A was deduced in 1931 by Karrer [ref 7] who proposed [ref 8] the name axerophthol (Axerophtol in German), based on its action in preventing the eye disease xerophthalmia. A second vitamin A, isolated by Morton and others, was called vitamin A2 [ref 9].
Recommendations for the nomenclature of the vitamins were published by IUPAC in 1960 [ref 10]. These included names for the three parent compounds - retinol, retinal and retinoic acid - as well as for their 3-dehydro analogues. A revised and enlarged version of these recommendations was published by the IUPAC-IUB Commission on Biochemical Nomenclature (CBN) in 1965 [ref 11]. With the publication by the IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and CBN of the 'Nomenclature of carotenoids' [ref 13] and by CNOC of 'Section F' (Natural products and related compounds) [ref 14] of the 'Nomenclature of Organic Chemistry' [ref 15] it was decided by JCBN that a separate document should be published extending section M-1 of the 1965 document. The present recommendations are based on drafts prepared by G. P. Moss in consultation with several active workers in the field and with CNOC.
Recommendations
Retinoids are a class of compounds consisting of four isoprenoid units joined in a head-to-tail manner. All retinoids may be formally derived from a monocyclic parent compound containing five carbon-carbon double bonds and a functional group at the terminus of the acyclic portion. To avoid confusion with previously used names in this field no parent hydrocarbon is named (see Ret-4.1).
The term vitamin A should be used as the generic descriptor for retinoids exhibiting qualitatively the biological activity of retinol. This term should be used in derived terms such as vitamin A activity, vitamin A deficiency, vitamin A antagonist [ref 16].
A stereoparent is a parent compound whose name implies stereochemistry, which will not need to be stated explicitly.
It is convenient to omit the explicit representation of C and H atoms in the skeletal formulae of retinoids as follows:
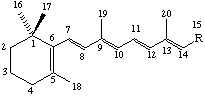
| (1) R = CH2OH | (6) R = CH2NH2 |
| (2) R = CHO | (7) R = CH=NOH |
| (3) R = CO2H | (8) R = CH=N[CH2]4CHNH2CO2H |
| (4) R = CH3 | (9) R = CO2C2H5 |
| (5) R = CH2OCOCH3 | (10) R- = 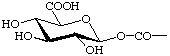 |
The compound (1) (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-l-yl)nona-2,4,6,8-tetraen-1-ol (see note 1), also known as vitamin A, vitamin A alcohol, vitamin A1, vitamin A1 alcohol, axerophthol or axerol, should be designated retinol (see note 2).
Note 1 The numbering system of the systematic name is different from above. See Ret-3.Note 2 WHO-approved nonproprietary names.
The compound (2) also known as vitamin A aldehyde, vitamin A1 aldehyde, retinene or retinene1 should be designated retinal (see note 1) or, if liable to be confused with the adjective retinal (pertaining to the retina), retinaldehyde (see note 2).
Note 1 Recommended for chemical usage.Note 2 Recommended for nutritional usage [ref 16].
The compound (3) also known as tretinoin (see note), vitamin A acid or vitamin A1 acid should be designated retinoic acid.
Note WHO-approved nonproprietary names.
The basic system of numbering is that shown in structures (1) to (10). This follows the system outlined in the carotenoid rules in Rule Car-4 and Rule Car-12.4 [ref 13].
Ret-4. Modification of Stereoparent
The hydrocarbon (4), also known as axerophthene, should be designated deoxyretinol (see note). This hydrocarbon cannot be considered as the stereoparent for the stem 'retin-' as it would then need to be called retinene, a name already used as a misleading synonym for retinal (Ret-2.2). A name such as retinane is inappropriate as it would imply a saturated hydrocarbon (Rule F-2.3 in [ref 14]).
Note The prefix deoxy may be either detachable or non-detachable (see Rule C-16.11 [ref 15]). In this document it is treated as a nondetachable prefix and, so that the locant 15 is not needed, it is always placed next to the stem name (cf. Rule C-16.32 [ref 15]).
Ret-4.2. Change of the Functional Group
Ret-4.2.1. Derivatives of the basic hydrocarbon Functional substitution at the 15 position of the basic hydrocarbon is denoted by the use of the group names retinyl (R is CH2-) or retinylidene (R is CH=), with retention of the original numbering of the basic hydrocarbon. For example (5) is retinyl acetate and (6) is retinylamine.
Ret-4.2.2. Derivatives of retinal A compound derived from retinal is named either as an aldehyde derivative (following Rule C-842, Rule C-922 or Rule C-982 in [ref 15]), or as a compound substituted by the bivalent radical retinylidene (Rule A-4.1 in [ref 15]). For example (7) is retinal oxime and (8) is N6-retinylidene-L-lysine.
Ret-4.2.3. Derivatives of retinoic acid Derivatives of retinoic acid named as carboxylic acid derivatives following Rule C-4.6 or Rule C-403 [ref 15]. For example (9) is.ethyl retinoate and (10) is 1-O-retinoyl-β-D-glucopyranuronic acid.
Ret-4.3. Changes in the Hydrogenation Level
Retinoids that differ in hydrogenation level from the corresponding stereoparents defined by Rule Ret-2 are named by use of the prefixes 'hydro' and 'dehydro' together with locants specifying the carbon atoms at which hydrogen atoms have been added or removed. These prefixes are nondetachable, and, if both occur in one name, are cited in the order dehydro before hydro (multiplying prefixes do not alfect the order); see Rule Car-6 [ref 13]. Examples:
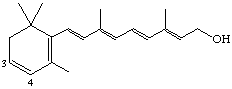
3,4-Didehydroretinol (also known as dehydroretinol [see note] or vitamin A2)
Note Recommended for nutritional usage [ref 16].
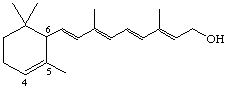
4,5-Didehydro-5,6-dihydroretinol (also known as α-vitamin A)
Ret-4.4. Substituted Retinoids
Substituted derivatives of retinoids are named by the use of prefixes according to the rules of general organic chemical nomenclature, see Rule C-0.1 [ref 15]. The use of suffixes is not possible since the stereoparents defined by Rule Ret-2 include a suffix concerned with the function at C-15. Examples:
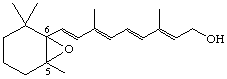
5,6-Epoxy-5,6-dihydroretinol (also known as hepaxanthin)
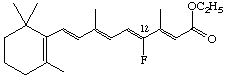
Ethyl 12-fluororetinoate
Fission of the ring, with addition of one or more hydrogen atoms at each terminal group thus created, is indicated by the prefix 'seco', the original retinoid numbering being retained (see Rule F-4.7 [ref 14]). Example:
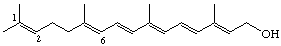
1,6-Seco-1,2-didehydroretinol (also known as γ-vitamin A)
Elimination of a CH3, CH2, CH or C group from a retinoid is indicated by the prefix 'nor', which is preceded by the locant of the carbon atom removed. When alternatives are possible, the locant selected is the highest possible, and the basic numbering of the retinoid is retained. The prefix is nondetachable (see F-4.2, F-4.4 [ref 14]). Note that this recommendation follows rules F-4.2 and F-4.4, rather than the carotenoid recommendation (Car-5.1 [ref 13]), which specifies the lowest possible locant. We propose that Car-5.1 should be changed to conform to the general practice when the carotenoid recommendations are next revised. Examples:
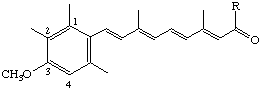
R = NHC2H5 N-Ethyl-3-methoxy-2-methyl-17-nor-1,2,3,4-tetradehydroretinamide (also known as motretinide [see note])
R = OC2H5 Ethyl 3-methoxy-2-methyl-17-nor-1,2,3,4-tetradehydroretinoate (also known as etretinate [see note])
Note WHO-approved nonproprietary names.
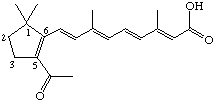
5-Acetyl-4,18-dinor-retinoic acid
The prefix retro (printed in italics) and a pair of locants indicate a shift by one position of the conjugated polyene system between the positions indicated by the locants. The first locant indicates the carbon atom that has lost a proton and the second the carbon atom that has gained one. This prefix is nondetachable (see Rule Car-9 [ref 13]). Examples:
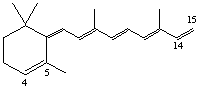
4,5-Didehydro-15,5-retro-deoxyretinol (also known as anhydro vitamin A)
Note. This semisystematic name is preferred over 14,15-didehydro-4,14-retro-deoxyretinol as it has the lower set of locants, see A-2.2, C-13.11 [ref 15].
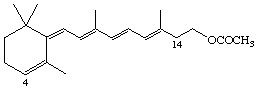
4,14-retro-Retinyl acetate
Other modifications may be indicated by the appropriate prefix. For example; cyclo (see Rule F-4.1 [ref 14]); homo (see Rule F-4.5 [ref 14]); hetero atoms (see Rule F-4.11 [ref 12], Rule B-4 and Rule C-0.6 [ref 14]); and additional rings (see Rule 2S Appendix [ref 17]).
The absolute configuration at chiral centres is designated by use of the RS convention, the symbols being placed, with the corresponding locants, before the retinoid name (see Rule E-4.9 [ref 18]). Examples:
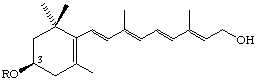
R = H (3R)-3-Hydroxyretinol
R = COCH3 (3R)-3-Acetoxyretinol
The stereoparent name defined by Rule Ret-2 implies that the polyene chain has the trans configuration about all double bonds unless the contrary is indicated. Following the designation of absolute configuration (if any), cis double bonds are indicated. The stereochemical prefixes E or Z (see Rule E-2 [ref 18]) may be used, and should always be used where cis or trans might be ambiguous. However it should be noted that substitution may result in a change from E to Z or vice versa even though there is no change in configuration of the stereoparent polyene chain. Hence it is recommended that the stereochemistry of all double bonds should be cited when this system is used. For example compound (9) is ethyl (all-E)-retinoate. Replacement of the hydrogen at C-12 by fluorine gives the compound used to illustrate rule Ret-4.4 which may be described as ethyl (7E,9E,11Z,13E)-12-fluororetinoate. Examples:
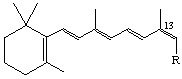
R=CH2OH 13-cis-Retinol or (7E,9E,11E,13Z)-retinol (also known as neovitamin A)
R = CO2H 13-cis-Retinoic acid or (7E,9E,11E,13Z)-retinoic acid (also known as isotretinoin [see note])
Note WHO-approved nonproprietary names.
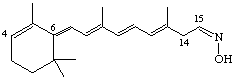
(6E,8E,10E,12E,15Z)-4,14-retro-Retinaloxime
Note that if it is necessary to indicate the stereochemistry of the 6(7)-double bond of a retro-retinoid this should be stated so that, for example, the compound given with Ret-4.7 is (6Z,8E,10E,12E)-4,14-retro-retinyl acetate.
1. Funk, C. (1912) J. State Med. 20, 341-368.
2. McCollum. E. V. & Kennedy, C. (1916) J. Biol. Chem. 24, 491-502.
3. Drummond, J. C. (1920) Biochem. J. 14, 660.
4. Wald, G. (1934) Nature (Lond.) 134, 65.
5. Morton, R. A. (1944) Nature (Lond.) 153,69-7l.
6. Morton, R. A. & Goodwin, T. W. (1944) Nature (Lond.) 153, 405-406.
7. Karrer, P., Morf, R. & Schoepp, K. (1931) HeIv. Chim. Acta, 14, 1431-1436.
8. v. Euler, H., Karrer, P. & Solmssen, U. (1938) Helv. Chim. Acta, 21, 211-222.
9. Edisbury, J. R., Morton, R. A. & Simkins, G. W. (1937) Nature (Lond.) 140, 234.
10. IUPAC Commission on the Nomenclature of Biological Chemistry (1960) Definitive rules for the nomenclature of vitamins, J. Am. Chem. Soc. 82, 5581-5581
11. IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Tentative rules, section on Trivial names of miscellaneous compounds of importance in Biochemistry, 1965, Arch. Biochem. Biophys. 118, 505-507 (1967); Biochem. J. 102, 15-16 (1967); Biochim. Biophys. Acta, 107, 1-4 (1965); Bull. Soc. Chim. Biol. (in French) 49, 332-335 (1967); Eur. J. Biochem. 2, 1-2 (1967); Hoppe-Seyler's Z. Physiol. Chem. (in German) 348, 266-268 (1967); IUPAC Inf. Bull. 25, 19-23 (1966); J. Biol. Chem. 241, 2987-2988 (1966); also on pages 212-213 in [ref 12].
12. International Union of Biochemistry (1978) Biochemical Nomenclature and Related Documents, The Biochemical Society, London.[Now available as the 1992 second edition.]
13. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Tentative rules for the nomenclature of carotenoids, Biochem. J. 127, 741-751 (1972) amended 151, 5-7 (1975); Biochemistry, 10, 4827-4837 (1971) amended 14, 1803-1804 (1975); Eur. J. Biochem. 25, 397-407 (1972) amended 57, 317-318 (1975) and, amendments incorporated, Pure Appl. Chem. 41, 405-431 (1975); also on pages 158-170 in [ref 12].
14. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of organic chemistry, Section F: Natural products and related compounds, Recommendations 1976, Eur. J. Biochem. 86, 1-8 (1978); also on pages 19-26 in [ref 12] and on pages 491-511 in [ref 15]. [Now available in a revised 1999 version.]
15. International Union of Pure and Applied Chemistry (1979) Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford.
16. International Union of Nutritional Sciences, Committee 1/I, Nomenclature (1978) Nutr. Abstr. Rev. 48A, 831-835.
17. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN),.The nomenclature of steroids, Revised tentative rules, 1967, Arch. Biochem. Biophys. 136, 13-35 (1970) amended 147, 4-7 (1971); Biochem. J. 113, 5-28 (1969) amended 127, 613-616 (1972); Biochemistry, 8, 2227-2242 (1969) amended 10, 4994-4995 (1971); Biochim. Biophys. Acta, 164, 453-486 (1968) amended 248, 387-390 (1971), Eur. J. Biochem. 10, 1-19 (1969) amended 25, 1-3 (1972) and, amendments incorporated, Pure Appl. Chem. 31, 285-322 (1972); also on pages 133-153 in [ref 12]. [Now available as the 1989 third edition.]
18 IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Rules for the nomenclature of organic chemistry, Section E: Stereochemistry, Recommendations 1974, Pure Appl. Chem. 45, 11-30 (1976); also on pages 1-18 in [ref 12] and on pages 4 73-490 in [ref 15].
Some Related Terpenoids Containing less than Twenty Carbon Atoms
The term carotenoid is usually restricted to terpenoids that retain the two central methyl groups of the parent C40 compound. However the definitions in Rule Car-1 and Rule Car-10 [ref 13] permit an extension of the apo nomenclature to include retinoids (e.g. retinal would be 15-apo-β-caroten-15-al) and a range of smaller molecules. Many of these compounds exhibit plant hormone action or are important essential oil components. The list of trivial names in this Appendix is given for convenience but is not intended as official recognition of these names.