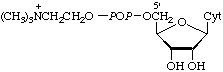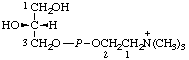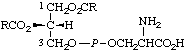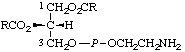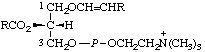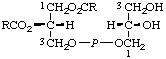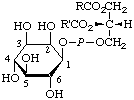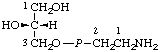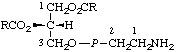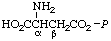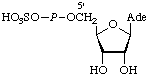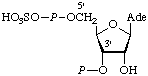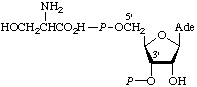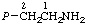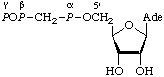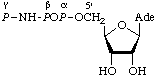IUPAC-IUB Commission on Biochemical Nomenclature (CBN)
Nomenclature of Phosphorus-Containing Compounds of Biochemical Importance
Recommendations, 1976
Continued from Recommendations and Table 1
Contents (continued)
Table 2. Representative oligophosphoric esters (oligophosphates)
Table 3. Representative bisnucleoside phosphates and cyclic phosphates
Table 4. Representative phospholipids (involving diesterified phosphoric acid)
Table 5. Phosphoric amides (phosphoramidic acids or amidophosphoric acids)
Table 6. Representative phosphoric anhydrides and fluorophosphates
Table 7. Representative C-phosphonates
Table 8. Adenosine 5'-triphosphate analogs
Table 2. Representative oligophosphoric esters (oligophosphates)
| Recommended names | Other names | Abbreviationsa | Structure |
| 1. | Farnesyl diphosphate;
farnesol diphosphate | Farnesyl trihydrogen
diphosphate;
farnesol (trihydrogen
diphosphate) | |  |
| 2. | Adenosine 5'-diphosphateb | 5'-Diphosphoadenosine;
5'-adenylyl phosphate | adenosine-5'PP;
Ado5'PP;
PPAdo;
ADP | 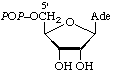 |
| 3. | 5-Phospho-α-D-ribosyl
diphosphatec;
α-D-ribosyl
diphosphate
5-phosphate | 5-O-Phosphono-α-D-ribosyl diphosphate;
5-phospho-α-D-ribofuranosyl
diphosphate;
1-diphospho-5-phospho-α-D-
-ribofuranose;
α-D-ribose 1-diphosphate
5-phosphate | PPRibPd;
PRib-PP | 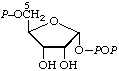 |
| 4. | Guanosine 2'(or 3')-diphosphate
5'-triphosphate | 2'(or 3')-Diphospho-5'-tri-
phosphoguanosine;
2'(or 3')-diphosphoguanylyl
triphosphate | pppGpp;
p3Gp2 | 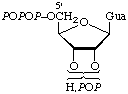 |
| 5. | Guanosine(5')tetra-
phospho(5')guanosine;
bis(guanylyl) diphosphate | P1,P4-Bis(5'-guanosyl)
tetraphosphate;
bis(5'-guanylyl) diphosphate | G(5')p4(5')G;
(ppG)2 | 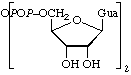 |
| 6. | 7-Methylguanosine(5')tri-
phospho(5')-2'-O-
-methylguanosinee | P1-(7-Methyl-5'-guanosinium-
-5'-yl) P3-(2'-O-methyl-5'-
-guanosyl) triphosphate;
7-methylguanosinium
5'-(2'-O-methylguanosine
5'-triphosphate) | m7G(5')ppp(5')Gm | 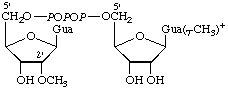 |
| 7. | Uridine(5')diphospho(1)-α-
-D-glucosec | Uridine diphosphate glucose;
uridine 5'-(α-D-glucopyranosyl
diphosphate);
α-D-glycopyranosyl
5'-uridylyl phosphate | UDP-Glc;
U5'pp1Glc;
UDPGf | 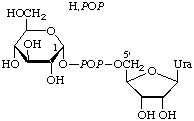 |
| 8. | Cytidine(5')diphosphocholinec | Cytidine diphosphate choline;
cytidine 5'-(choline
diphosphate);
choline (5'-cytidylyl
phosphate) | CDP-choline;
CDP-Cho | 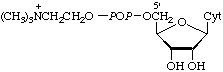 |
| 9. | Adenosine(5')diphospho(5)-β-
-D-ribosec,g | Adenosine diphosphate
ribose;
adenosine 5'-(β-D-ribose
5-diphosphate) | ADP-Rib;
A5'pp5Rib;
(Rib5)ppA | 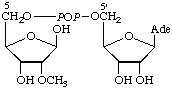 |
| 10. | 2'-(5-Phospho-β-D-ribosyl)adenosine
5'-phosphatec,g | 2'-(5-Phospho-β-D-ribofur-
anosyl)adenosine
5'-phosphate;
2'-(5-phospho-β-D-ribofur-
anosyl)-5'-adenylic acid | AMP(P-Rib);
P-Ado(P-Rib);
pA2'(pRib);
pRib(l-2')pA;
iso(ADP-Rib);
ψADP-Ribh | 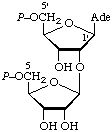 |
a The symbol Rib for the ribose residue is defined in ref 3. Other symbols are defined in ref 9, especially P or p = phosphoric residue.
b Triphosphates are treated similarly. Analogs of ATP are further delineated in Table 6. Other nucleoside terms may replace "adenosine," "adenylyl," and "Ado" (ref 9).
c Locants and descriptors are custoinarily omitted for the isomers shown, so that the terms may be written as, e.g., phosphoribosyl diphosphate, uridinediphosphoglucose (compound 7).
d The abbreviation PPRP is seen often in the literature; it is not recommended.
e Bis(nucleotides) of this type, including the methylation as shown, appear to be the common 5'-terminus of many, if not all, of the RNAs of RNA-containing viruses. The use of 7-methyl guanosine instead of the more correct 7-methyl-7-guanosinium is common.
f Common, but can be ambiguous in context with galactose. Glc for glucose is preferred.
g Cleavage of "poly(ADP-Rib)" at the 2' linkage of the adenosine moieties gives compound 9, and between the phosphoric residues gives compound 10. Although compound 10 is not an oligophosphoric ester and does not really belong in this table, it is included because it is most often encountered in conjunction with the isomeric compound 9.
h Disfavored; given here because of occurrence in the literature. (ψ has other meanings in this context.)
Table 3. Representative bisnucleoside phosphates and cyclic phosphates (ref 3, 9).
| Recommended namea | Other nameb | Abbreviations | General structurec |
| 1. | Adenylyl (3'-5')cytidine | Cytidylyl(5' 3')adenosine 3')adenosine | A-C;
Ado-3'P5'-Cyd | 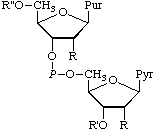 |
| 2. | Adenylyl(3'-5')cytidine
3'-phosphate | 3'-Phosphocytidylyl(5' 3')adenosine 3')adenosine | A-Cp;
Ado-3'P5'-CydP |
| 3. | 5'-Phosphoadenylyl(3'-5')cytidine
3'-phosphate | 3'-Phosphocytidylyl(5' 3')adenosine 3')adenosine
5'-phosphate | pA-Cp;
PAdo-3'P5'-CydP |
| 4. | 5'-Phosphodeoxyadenylyl(3'-5')-
thymidined | Thymidylyl(5' 3')deoxyadenosine 3')deoxyadenosine
5'-phosphate | pdA-dT;
d(pA-T);
P-dAdo-3'P5'-dThd |
| 5. | 5'-Triphosphoguanylyl(3'-5')cytidine | Cytidyly1(5' 3')guanosine 3')guanosine
5'-triphosphate | pppG-C |
| 6. | Cytidine 2',3'-phosphate | Cytidine 2'3'-(cyclic)phosphatee | Cyd>P;
Cyd-2',3'-P;
C>p |
| 7. | Adenosine 3',5'-phosphate | Adenosine 3',5'-(cyclic)phosphatee | Cyclic AMP;
cAMP;
Ado-3',5'-P |
a The infix (3'-5') may be omitted if no ambiguity may arise.
b Many other names, including permutations of those given, are possible, e.g., adenosine(3')-phospho(5')cytidine and 5'-(3'-adenyiyl)cytidine for compound 1.
c General structure for entries 1-5: R = OH in entries 1, 2, 3, and 5; H in 4. R' = H in 1, 4, and 5; P in 2 and 3. R" = Hin 1 and 2; P in 3 and 4; POPOP in 5.
d The prefix "ribo" precedes "thymidine" when 5-methyluridine is meant. The symbols Thd and T indicate the latter; dThd and dT represent thymidine (ref 3, 9).
e "(Cyclic)" is redundant when locants are given in compounds 6 and 7, but is helpful. It is necessary when, in repeated usage, the locants are omitted.
Table 4. Representative phospholipids (involving diesterified phosphoric acid)
| Recommended namea,b | Other names | Abbreviationsc | Structure |
| 1. | sn-Glycero(3)phosphocholine | Glycerol choline phosphate | GroPCho | 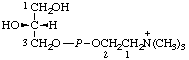 |
| 2. | (3-sn-Phosphatidyl)choline | 1,2-Diacyl-sn-glycero(3)phosphocholine;
lecithind | PtdCho;
acyl2GroPCho |  |
| 3. | (3-sn-Phosphatidyl)-L-serine | 1,2-Diacyl-sn-glycero(3)-L-phosphoserine;
cephalind | PtdSer;
acyl2GroPSer | 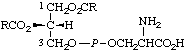 |
| 4. | (3-sn-Phosphatidyl)ethanolamine | 1,2-Diacyl-sn-glycero(3)phospho-
ethanolamine;
cephalind | PtdEtn;
acyl2GroPEtn | 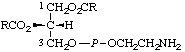 |
| 5. | 2-Acyl-1-(1-alkenyl)-sn-
glycero(3)phosphocholine | Plasmenylcholinee;
plasmalogen | | 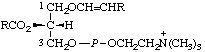 |
| 6. | 1-(3-sn-Phosphatidyl)-sn-
glycerol | 1,2-Diacyl-sn-glycero(3)phospho(1)-sn-
-glycerol | PtdGro | 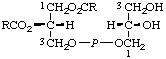 |
7.
 | 1-(3-sn-Phosphatidyl)-D-myo-
inositol | 1,2-Diacyl-sn-glycero(3)phospho(1)-D-myo-
inositol;
phosphoinositided | PtdIns | 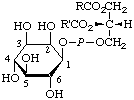 |
8.
 | 1-(3-sn-Phosphatidyl)-D-myo-
inositol 4-phosphate | 1,2-Diacyl-sn-glycero(3)phospho(1)-D-myo-
inositol 4-phosphate;
1,2-diacyl-sn-glycero(3)phospho(1)-4-
-phospho-D-myo-inositol;
phosphoinositide 4-phosphated;
diphosphoinositided | PtdIns4P;
PtdIns-4-P | 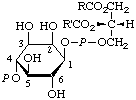 |
9.
 | 1-(3-sn-Phosphatidyl)-D-myo-
inesitol 4,5-bis(phosphate) | 1,2-Diacyl-sn-glycero(3)phospho(1)-D-myo-
inositol 4,5-bis(phosphate);
1,2-diacyl-sn-glycero(3)-phospho(1)-4,5-bis-
(phospho)-D-myo-inositol;
phosphoinositide 4,5-bis(phosphate)d;
triphosphoinositided | PtdIns(4,5)P2;
PtdIns-4,5-P2 | 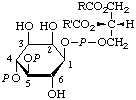 |
| 10. | sn-Glycero(3)-2-
-phosphonoethylaminef | sn-Glycerol 3-[(2-aminoethyl)phosphonate] | GroPEtNH2 | 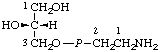 |
| 11. | (3-sn-Phosphatidyl)ethylaminef | 1,2-Diacyl-sn-glycero(3)-2-
-phosphonoethylamine | PtdEtNH2 | 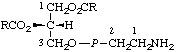 |
a Stereospecific numbering denoted by sn is defined in ref 8. If fully defined in a paper, sn and various locants and descriptors may be omitted from the recommended names and abbreviations. The infix "phospho" replaces "phosphoryl" and "phosphinico," which have been used in the past (ref 2, 3) (see Sections 1 and 4 of the text).
b Phosphatidyl = 1,2-diacyl-sn-glycero(3)phospho, which may replace it when desired. For O-alkenyl- and O-alkyl-substituted glycero compounds ("lyso" compounds), see entry 5 and ref 5.
c The symbols Ptd for phosphatidyl, Gro for glycerol, Cho for choline, Ser for serine, Etn for othanolamine, Ins for inositol, and P for a phosphoric residue are defined in ref 5.
d Trivial names occasionally used in the past; not recommended. Included here only for reference.
e Plasmenyl and plasmanyl are defined in ref 5.
f Phosphonic derivatives, containing a P-C bond (compare entry 4, also Table 7).
Table 5. Phosphoric amides (phosphoramidic acids or amidophosphoric acids)
| Recommended namesa | Other namesb,c | Structure |
| 1. | Phosphocreatine | Nω-Phosphonocreatine;
N-(N-Phosphonoamidino)sarcosine |  |
| 2. | Phosphoglycocyamine | Nω-Phosphonoglycocyamine;
N-phosphonoguanidinoacetic acid;
N-(N-phosphonoamidino)glycine |  |
| 3. | Phosphoguanidine | N-Amidinophosphoramidate;
N-amidinophosphoramidic acid |  |
| 4. | pros-Phosphohistidined;
τ-phosphohistidine | 3(1)- Phosphonohistidinec | 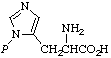 |
| 5. | tele-Phosphohistidined;
τ-phosphohistidine | 1(3)-Phosphonohistidinec | 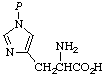 |
a See Section 6 of the text for a discussion of the reasons for not using the "phosphate" form of name for the phosphoric amides. P-Creatine and creatine-P are valid for abbreviation purposes, on the assumption that the hyphen indicates a covalent bond;
names such as creatine phosphate do not indicate a covalent bond.
b The symbol ω is used to mean the NH2 terminal group.
c The prefix "phosphonato" may be used to indicate an ionic form (ref 1, Rule 5.52).
d For definition of pros (π) and tele (τ) locants, see ref 10, 12.
Table 6. Representative phosphoric anhydrides and fluorophosphatesa
| Recommended names | Other names | Abbreviations | Structure |
| 1. | Acetyl phosphate | Monoacetyl phosphate;
acetic phosphoric monoanhydride | Ac-P | CH3CO2-P |
| 2. | β-Aspartyl phosphate | Mono-β-aspartyl phosphate;
β-aspartic phosphoric monoanhydride | Asp(βP)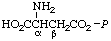 |
| 3. | Carbamoyl phosphateb | Monocarbamoyl phosphate;
carbamic phosphoric monoanhydride | Cbm-P | H2NCO2-P |
| 4. | Adenosine
5'-phosphosulfate | Adenosine 5'-P-phosphatosulfatec;
5'-adenylyl sulfate;
5'-adenytic sulfuric monoanhydride | APSd;
AdoPS | 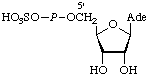 |
| 5. | Adenosine 3'-phosphate
5'-phosphosulfate;
3'-phosphoadenosine
5'-phosphosulfate | Adenosine 3'-phosphate 5'-P-phosphatosulfatec;
3'-phospho-5'-adenylyl sulfate;
3'-phospho-5'-adenylic sulfuric monoanhydride | PAPSd;
PAdoPS | 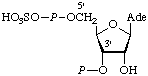 |
| 6. | Seryl adenylate | 1-O-(5'-Adenylyl)serine;
adenosine(5')phospho(1)serine | AMP-Ser;
Ser-P-Ado | 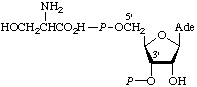 |
| 7. | 3-Phosphoglyceroyl
phosphatee | 3-O-Phosphonoglyceric phosphoric
monoanhydride;
(glyceroyl phosphate) 3-phosphate | Gri(1,3)P2f |  |
| 8. | Diisopropyl fluorophosphate | Diisopropyl phosphorofluoridate | iPr2P-Fg | [(CH3)2CHO]2P(O)F |
a See Sections 3 and 7 in text.
b "Carbamyl," which is often used, is not in accord with the Organic Rules (ref 7 Rule 431.2).
c See Rules of Inorganic Nomenclature (ref 6, Rule 4.211).
d Commonly used in the literature; Ado form is preferred for A and P and S for the acid residues.
e See entry 16 in Table 1.
f See footnote b in Table 1.
g Equivalent to DIPF, FDIP, DFP, and Dip-F (ref 10).
Table 7. Representative C-phosphonates
| Recommended name | Other namesa | Structure |
| 1. | (2-Aminoethyl)phosphonic acidb | 2-Phosphonoethylamine;
ciliatine | 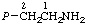 |
| 2. | (2-Oxoethyl)phosphonic acid | (Formyimethyl)phosphonic acid;
phosphonoacetaldehyde | P-CH2CHO |
a The prefix "phosphonato" may be used to indicate an ionic form (ref 1, Rule 5.52).
b See also entries 10 and 11 in Table 4.
Table 8. Adenosine 5'-triphosphate analogsa
| Recommended name | Other namesb | Abbreviations | Structure |
1.
 | Adenosine
5'-α,β-μ-methylenetri-
phosphate;
adenosine
5'-[α,β-methylene]tri-
phosphateAdenosine (5' O1)-1,2-μ- O1)-1,2-μ-
-methylenetriphosphate;
adenosine (5' P1)-1,2-μ- P1)-1,2-μ-
-methylenetriphosphate | AdoP[CH2]PP;
pp[CH2]pA | 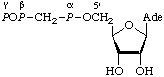 |
2.
 | Adenosine
5'-β,γ-μ-imidotri-
phosphate;
adenosine
5'-[β,γ-imido]tri-
phosphate | Adenosine (5' O3)-1,2-μ- O3)-1,2-μ-
-imidotriphosphate;
adenosine (5' P3)-1,2-μ- P3)-1,2-μ-
-imidotriphosphate;
5'-adenylyl imidodiphosphate;
5'-adenylyl iminodiphosphonatec | AdoPP[NH]P;
p[NH]ppA | 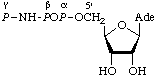 |
3.
 | Adenosine
5'-γ-thiotri-
phosphate | Adenosine (5' O3)-1- O3)-1-
-thiotriphosphate;
adenosine (5'-P3)-1-
-thiotriphosphate | AdoPPP[S];
[S]pppA;
ATP[S] | 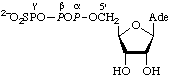 |
a See Section 5 in text.
b Adaptation of principles of inorganic nomenclature for isopolyanions (ref 6, Rule 4.15).
c "Iminodiphosphonate", is derived from organic nomenclature principles (ref 7, Rules B-15.1, C-815.1).
Return to IUPAC chemical nomenclature home page
Return to IUBMB biochemical nomenclature home page