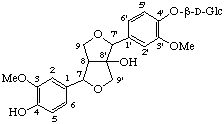
4,4',9'-trihydroxy-3,3',5,5'-tetramethoxy-2,7'-cyclolign-7-en-9-oic acid
Continued from LG-3 Changes in Hydrogenation Level
Contents
Functional groups and other substituents are expressed as in the general recommendations for organic compounds. Where possible the preferred group is cited as a suffix and all other groups are expressed by means of prefixes. For the more commonly encountered functional groups the order of preference as a suffix is carboxylic acid, lactone, ester, ketone and alcohol. When the suffix starts with a vowel the "e" of lignane is elided. All groups other than that expressed as a suffix are cited as prefixes which are quoted in alphabetical order ignoring multiplying affixes such as di-, tri-, tetra-, etc. If there is a choice of locations for a functional group preference is given in the order:
(a) unprimed numbersLG-4.1 Carboxylic Acids(b) lower numbers for principal functional group i.e. the group named by a suffix
(c) lower numbers for locants for prefixes when the locants are considered as a set
(d) lower numbers for locants for prefixes in the order of citation
When a methyl group of a lignan or neolignan is converted into a carboxy group this is indicated by the suffix -oic acid with the locant of this position.
Example:

Lactones are named by changing the ending -ic acid of the corresponding acid to -lactone preceded by the locant of the acid group and then the locant of the hydroxy group which forms the lactone in that order. The preference for the carbonyl group to be unprimed is contrary to common practice with symmetric skeletons [e.g. steganone (see LG-5.3) and enterolactone (see LG-5.5)]. The preference for the unprimed number assists comparison with related compounds such as other carboxylic acid derivatives and stereochemistry (see LG-5.3). When the lactone group is part of a bridge [e.g. gonisin D (see LG-5.3)] one of the oxygen atoms is included in the bridge and the other oxygen atom of the carbonyl group is treated as a "ketone" (see LG-4.4).
Examples:
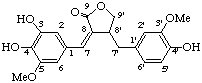

7-hydroxy-3',4',5'-trimethoxy-4,5-methylenedioxy-2,7'-cyclolignano-9',9-lactone
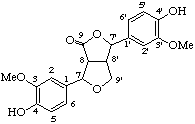
4,4'-dihydroxy-3,3'-dimethoxy-7,9'-epoxylignano-9,7'-lactone
Esterification of a carboxylic acid (see LG-4.1) is indicated by changing the suffix -oic acid to -oate and adding the name of the alkyl group in front of the name.
Example:
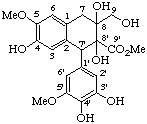
Esterification of an alcoholic or phenolic hydroxy group (see LG-4.5) is indicated by replacing the terminal -e of lignane, neolignane or oxyneolignane by -yl, (or by the prefix -diyl, -triyl, etc.) with the locant of the associated position and the anionic form of the acyloxy group. If there is a preferred group such as a carboxylic acid, lactone or ester of a carboxy group the esterified hydroxy group is indicated by an acyloxy prefix.
Examples:
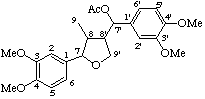
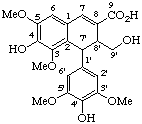
8-hydroxy-3',5-dimethoxy-2,7'-cyclolignane-4,4',9,9'-tetrayl tetraacetate
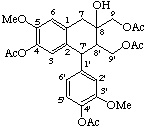
Ketones are named by the suffix -one (or the prefix oxo-, if a preferred group is used as a suffix). If the ketone is cited as a suffix at one of the ring atoms C-2 to C-6, and it is necessary to identify double bond locations this is shown by added hydrogen cited in brackets after the locant of the ketone.
Examples:
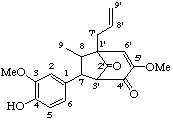
4-hydroxy-3,5'-dimethoxy-3',7-cyclo-8,1'-neolign-8'-ene-2',4'(3'H)-dione
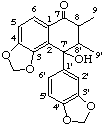
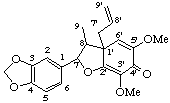
7'-hydroxy-3,4:3',4'-bis(methylenedioxy)-2,7'-cyclolignan-7-one
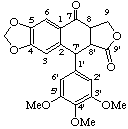
3',4',5'-trimethoxy-4,5-methylenedioxy-7-oxo-2,7'-cyclolignano-9',9-lactone
Alcohols or phenols are named by the suffix -ol; or by the prefix hydroxy- if there is a preferred group used as a suffix.
Examples:
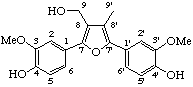
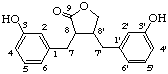
3,3'-dihydroxylignano-9,9'-lactone
LG-4.6 Ethers and Alcohol or Phenol Derivatives
Ethers can only be named by means of an alkoxy prefix. Sugar derivatives are named by a glycosyloxy prefix. Esters of alcoholic or phenolic lignanes, neolignanes or oxyneolignanes are considered in LG-4.3.
Example: