Fused Ring and Bridged Fused Ring Nomenclature
(IUPAC Recommendations 1998)
FR-4.6 Omission of Locants
Continued from FR-4.5 Choice of Locants
Contents of Section
FR-4.6 Omission of locants
FR-4.6.1 Unnecessary locants
FR-4.6.2 Locants of peripheral fusion carbon atoms of a component
FR-4.7 Elision
FR-4.8 Heteroatom locants of components
FR-4.9 Treatment of identical attached components
FR-4.9.1 Additional components attached to a system with a multiplicative prefix
FR-4.9.2 Groups of identical components with identical fusion
Reference for this Section
Continue with FR-5 Numbering.
FR-4.6 Omission of locants
FR-4.6.1 Unnecessary locants
If there is no ambiguity the numerical and/or letter locants may be omitted for a system with only first-order attached components.
Examples:
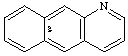
benzo[g]quinoline
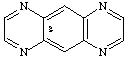
pyrazino[g]quinoxaline
CAS and Beilstein name pyrazino[2,3-g]quinoxaline
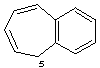
5H-benzo[7]annulene
CAS and Beilstein name 5H-benzocycloheptene
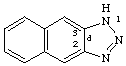
1H-naphtho[2,3][1,2,3]triazole
CAS name 1H-naphtho[2,3-d]triazole
Beistein name 1H-naphtho[2,3-d][1,2,3]triazole
Locants are also omitted when there is no ambiguity for the fusion of terminal attached components.
Example:
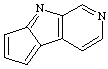
cyclopenta[4,5]pyrrolo[2,3-c]pyridine
When locants are required for the fusion of a higher-order component then all locants for the linking component must be cited.
Example:
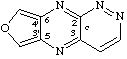
furo[3',4':5,6]pyrazino[2,3-c]pyridazine
FR-4.6.2 Locants of peripheral fusion carbon atoms of a component
The numerical locants of peripheral fusion carbon atoms of a component are omitted with an ortho- and peri-fused system.
Examples:
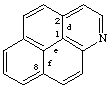
naphtho[2,1,8-def]quinoline
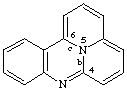
quinolizino[4,5,6-bc]quinazoline
Both terminal fusion atom locants for ortho-fusion must be cited even if one is a fusion atom.
Example:
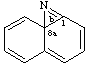
naphtho[1,8a]azirine
FR-4.7 Elision
Vowels are not elided in fusion names, however see FR-2.2.8.
Note
The previous edition of these rules included elision (see footnote to A-21.4 ref 3) which is still used by CAS. in the following way.
When acenaphtho-, benzo-, or naphtho- is followed by a component which starts with a vowel the 'o' is elided. Similarly the 'a' of cycloalka- is elided under these conditions. In all other cases the 'o' or 'a' is retained.
Examples:
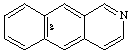
CAS name benz[g]isoquinoline
IUPAC name benzo[g]isoquinoline
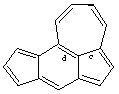
CAS name cyclohept[cd]-s-indacene
IUPAC name cyclohepta[cd]-s-indacene
If there is more than one first-order attached component, and at least one second-order attached component, elision between the second-order and the first-order attached component is applied after determination of the alphabetical order of the components (see FR-3.6).
Example:
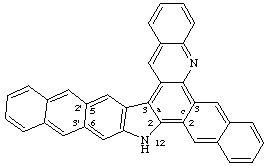
CAS name 12H-naphtho[2,3-c]naphth[2',3':5,6]indolo[3,2-a]acridine
even though naphthindolo is alphabetically before naphtho
IUPAC name 12H-naphtho[2,3-c]naphtho[2',3':5,6]indolo[3,2-a]acridine
FR-4.8 Heteroatom locants of components
When a component requires the citation of locants (e.g. in a Hantzsch-Widman name) these are always quoted in square brackets. Note that these numbers in brackets only refer to the numbering of the component and have no significance in the final numbering of the complete fused ring system.
Examples:
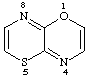
[1,4]thiazino[3,2-b][1,4]oxazine
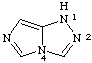
1H-imidazo[5,1-c][1,2,4]triazole
Note
In the previous version of these rules (See B-3.1, ref 3) any locants of a component which are cited in the name and are the same as that of the final system (see FR-5.3 and FR-5.4) are not enclosed in brackets.
CAS still does not use brackets when the locants of the heteroatoms of the component correspond to those of the whole fused ring system. Thus the CAS index names for the above examples are [1,4]thiazino[3,2-b]-1,4-oxazine and 1H-imidazo[5,1-c]-1,2,4-triazole although in the latter case the triazole was numbered anticlockwise to derive the fusion locants and clockwise afterwards to assign the system locants.
FR-4.9 Treatment of identical attached components
When there are two or more components which are identical and both fused to a parent or attached component this is indicated by the use of the prefix di-, tri-, etc. (or bis-, tris-, etc. to avoid ambiguity). This multiplicative prefix is not considered in deciding the alphabetical order of components attached at the same level (see FR-3.6).
If a complete set of locants are used for first-order attached components fused to the parent component these are cited together separated by a colon. If abbreviated sets of locants are used the letters are separated by a comma. If complete sets of locant are used for second-order attached components fused to a first-order attached component, or for higher-order cases, the locants are cited together separated by a semicolon. If abbreviated sets are used they are separated by a colon. (See note 1)
To distinguish between the two or more components of the same order the locants of the second component are primed (or double primed if the first is primed, etc.), the third double primed etc.
Examples:
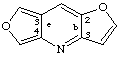
difuro[3,2-b:3',4'-e]pyridine
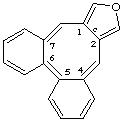
dibenzo[4,5:6,7]cycloocta[1,2-c]furan
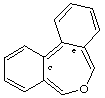
dibenzo[c,e]oxepine
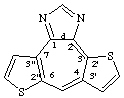
dithieno[2',3':3,4;2",3":6,7]cyclohepta[1,2-d]imidazole
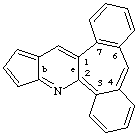
cyclopenta[b]dibenzo[3,4:6,7]cyclohepta[1,2-e]pyridine
If the use of di-, tri-, etc. might be ambiguous bis-, tris-, etc. are used. This applies when di-, tri-, etc. prefixes might be used to name the component (e.g. Hantzsch-Widman name).
Example:
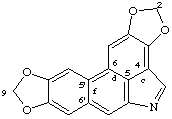
2H,9H-bis[1,3]benzodioxolo[4,5,6-cd:5',6'-f]indole
Beilstein name [1,3]dioxolo[4',5':4,5]benzo[1,2-f][1,3]dioxolo[4',5':5,6]benzo[1,2,3-cd]indole
Note
Apart from the use of a hyphen with a full set of locants for fusion of the first-order attached component to a parent component the punctuation is in the order comma, colon, semicolon. See examples above. The semicolon separates sets which use a colon; the colon separates sets which use a comma or hyphen; and the comma is used if only one punctuation mark is needed.
Beilstein uses the hyphen as above. A colon is used for fusion of attached components. A semicolon is used to separate fusion descriptors which already contain a colon or comma e.g. difuro[3,2-b;3',4'-e]pyridine and dibenzo[4,5;6,7]cycloocta[1,2-c]furan (cf. above).
FR-4.9.1 Additional components attached to a system with a multiplicative prefix
Fusion of a higher-order attached component to a system named with a multiplicative prefix requires each set of attached components to be specified separately.
Example:
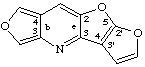
furo[3,4-b]furo[3',2':4,5]furo[2,3-e]pyridine
FR-4.9.2 Groups of identical components with identical fusion
When two or more groups of identical components (including identical fusion between these components) are fused to another component this is indicated by the use of bis-, tris-, etc. with the group of components in round brackets.
Example:
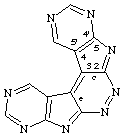
bis(pyrimido[5',4':4,5]pyrrolo)[2,3-c:3',2'-e]pyridazine
CAS name bispyrimido[5',4':4,5]pyrrolo[2,3-c:3',2'-e]pyridazine
Reference for this Section
3. IUPAC Nomenclature of Organic Chemistry, Sections A and B, 1st edition, 1958; 2nd edition, 1966; 3rd edition (combined with section C), 1971; 4th edition (combined with sections C, D, E, F and H), 1979.
Continue with FR-5 Numbering.
Return to home page for Fused Ring Nomenclature.