Nomenclature Committee of the International Union of Biochemistry (NC-IUB)
https://iupac.qmul.ac.uk/cyclitol/myo.html
World Wide Web version prepared by G. P. Moss
School of Biological and Chemical Sciences, Queen Mary and Westfield College,
Mile End Road, London, E1 4NS, UK
e-mail g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version prepared with the expert advice of S.J. Angyal (Australia), C.P. Downes (U.K.), F. Eisenberg, Jr (U.S A.), R. F. Irvine (U.K.), R. H. Michell (U K.), R. Parthasarathy (India) and V. I. Shvets (U.S.S.R.) [see Biochem.J., 1989, 258, 1-2; Eur. J. Biochem., 1989, 180, 485-486; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 156-157; Copyright IUBMB; reproduced with the permission of IUBMB]. If you need to cite these rules please quote this reference as their source.
Any comments should be sent to any member of the Committee
A relaxation of previous recommendations on the numbering of the atoms of myo-inositol is suggested: substituents need not necessarily be numbered so that the smallest possible locant is used. This allows an alternative designation to be used, when authors wish, to bring out structural relationships.
1. Introduction
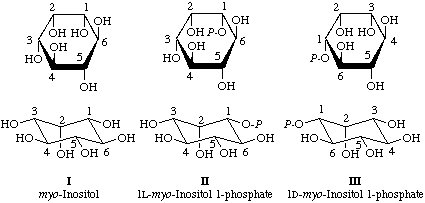
Present recommendations on the numbering of cyclitols [1] lead to the designations and numbering shown in structures I, II and III, namely myo-inositol, 1L-myo-inositol 1-phosphate (the compound formed from glucose 6-phosphate in the biosynthesis of inositol), and 1D-myo-inositol 1-phosphate (the constituent of phospholipids and inositol polyphosphates).

Thus phosphorylation on O-1 of myo-inositol leads to 1L-myo-inositol 1-phosphate, whereas phosphorylation on O-3 alters the numbering of the carbon atoms, reversing C-1 to C-3, and so leads to 1D-myo-inositol 1-phosphate. The reason for this is that recommendation I-4 [1] first allocates the locants 1, 2, 3 and 5 to the four hydroxyl groups that are on one side of the ring, without specifying the starting point. It then allocates the lowest possible locant to the substituted one, so that phosphates II and III are both 1-phosphates.
The prefix D or L is then assigned according to recommendation I-10 [1]: if the ring numbering appears clockwise when the substituent at the lowest numbered chiral centre (here C-1) is away from the viewer (i.e. when the rings drawn are viewed from above), the compound is 1D. (The 1 indicates that C-1 is the chiral centre used).
For achiral compounds, e.g. myo-inositol itself, the numbering is defined as that that gives 1L. Hence such compounds, e.g. myo-inositol with identical substituents on positions 1, 3, 4 and 6, have to be allotted 1L or 1D designations to give the locants specific meanings.
2. Proposal for Stereospecific Numbering
Klyashchitskii et al. [2] have suggested stereospecific numbering for myo-inositol, as has proved successful for glycerol derivatives [3], and Parthasarathy and Eisenberg [4] have advocated such a system. It would overcome the difficulty that phosphorylation at O-1 gives 1L-myo-inositol 1-phosphate whereas phosphorylation at O-3 alters the numbering of the carbon chain to give 1D-myo-inositol 1-phosphate.
Such stereospecific numbering would be a minimal change from existing practice, and would leave the numbering of all the inositol bis- and trisphosphates unchanged; it would also change the name of the product of the reaction catalysed by the enzyme classified as EC 5.5.1.4 (currently with recommended name myo-inositol 1-phosphate synthase) to an inositol 3-phosphate instead of 1L-myo-inositol 1-phosphate, and its relationship to the other derivatives would be clearer. Despite these advantages, we are advised that stereospecific numbering would be confusing to many who are not concerned with metabolic pathways, would obscure enantiomeric relationships, and would disrupt existing usage and indexing.
3. Present Recommendations
3.1. Relaxation of lowest-locant rule
The advantages of stereospecific numbering can be obtained by relaxing the rule that a substituent must have been lowest possible locant. Thus a compound that by the priority rules belongs to the 1L series may be given the 1D numbering if this shows relationships that the author wishes to stress. As the prefix 1D will be present, no ambiguity results. We suggest that this should be done whenever it is convenient in biochemical work. Thus 1L-myo-inositol 1-phosphate may be called 1D-myo-inositol 3-phosphate if it is desired to point out the 1,3 relationship to 1D-myo-inositol 1-phosphate. Consider, for example the following metabolic pathway [5, 6]:
1D-myo-inositol 1,4,5-trisphosphate
1D-myo-inositol 1,3,4,5-tetrakisphosphate
1D-myo-inositol 1,3,4-trisphosphate
1L-myo-inositol 1,6-bisphosphate
It is not immediately obvious that the last step is hydrolysis at C-1, but this becomes easier to follow if the pathway is written as follows (as was done in the original work [5, 6]):
1D-myo-inositol 1,4,5-trisphosphate
1D-myo-inositol 1,3,4,5-tetrakisphosphate
1D-myo-inositol 1,3,4-trisphosphate
1D-myo-inositol 3,4-bisphosphate
3.2. The symbol Ins
The symbol Ins was previously given to inositol [3]. Its use is largely conflned to biochemical work, and we further suggest that it should be taken to mean myo-inositol with the numbering of the 1D configuration unless the prefix L is explicitly added. (This is similar to recommendation 3AA-3.3 in the recommendations on amino-acid nomenclature [7] that in biochemical work the symbol Ala refers to L-alanine). Hence the above pathway may be symbolized as follows:
Ins(1,4,5)P3Ins(1,3,4,5)P4
Ins(1,3,4)P3
Ins(3,4)P2
4. Numbering of Atoms in the Chair Conformation
As the numbering of atoms in myo-inositol can become especially confusing when the Haworth projections used for defining the different forms are converted into diagrams representing the normal chair conformations of the molecules, we drawn attention to 'Agranoff's turtle' [9], a useful mnemonic device for avoiding the confusion.
5. References
1. IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of cyclitols. Recommendations 1973. Biochem. J. 153, 23-31 (1976); Eur. J. Biochem. 57, 1-7 (1975); Pure Appl. Chem. 37, 285-297 (1974); also pp. 196-202 in [8]. [Also in Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 149-155.]
2. Klyashchitskii, B. A., Shvets, V. I. & Preobrazhenskii, N. A. (1969) Chem. Phys. Lipids 3, 393-400.
3. IUPAC-IUB Commission on Biochemical Nomenclature (CBN). The nomenclature of lipids. Recommendations 1976. Biochem. J. 171, 21-35 (1978); Eur. J. Biochem. 79, 11-21 (1977); Hoppe-Seyler's Z. Physiol. Chem. 358, 617-631 (1977); also pp. 122-132 in [8]. [Also in Chem. Phys. Lipids, 1978, 21, 159-173; J. Lipid Res., 1978, 19, 114-128; Lipids, 1977, 12, 455-468; Mol. Cell. Biochem., 1977, 17, 157-171; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 180-190.]
4. Parthasarathy, R. & Eisenberg, E., Jr (1986) Biochem. J. 235, 313-322
5. Shears, S. B., Storey, D. J., Morris, A. B., Cubitt, A. B., Parry, J. B., Michell, R. H. & Kirk, C. J. (1987) Biochem. J. 242, 393-402.
6. Irvine, R. F., Letcher, A. J., Lander, D. J., Heslop, J. P. & Serridge, M. J. (1987) Biochem. Biophys. Res. Commun. 143, 353-359.
7. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations 1983. Biochem. J. 219, 345-373 (1984); Eur. J. Biochem. 138, 9-37 (1984); Int. J. Pept. Prot. Res. 24, following p. 84 (1984); J. Biol. Chem. 260, 14-42 (1985); Pure Appl. Chem. 56, 595-624 (1984); Spec. Period. Reps Amino Acids Pept. 16. 387-410 (1985). [Also in Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 39-69.]
8. International Union of Biochemistry (1978) Biochemical nomenclature and related documents, The Biochemical Society, London. [Now as the 1992 edition]
9. Agranoff, B. W., Trends Biochem. Sci. (1978) 3, N283-N285.