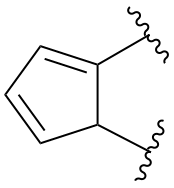
Replace structure with:

Division VIII Chemical Nomenclature and Structure Representation Division
World Wide Web version Prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
For additional minor corrections click here Characters deleted from the printed text or which are modified are marked in red on the original text. Characters added to the corrected text are marked in red too.
Page xxxii, entry 7 (g), lines 1/2. [modified 3 March 2021]
For ...preferred IUPAC names; the preferred prefix is '’carbonimidamido'.
read ..preferred IUPAC names but may be used in general nomenclature; the preferred prefix is 'carbamimidoylamino'.
RO minor correction 13.2.2021 change to BBminor
Page xxxiii, Changes 7 (l), lines 1-4. [corrected 21 August 2019]
For ...from ‘triazane’ and ‘tetrazane’ are named systematically as hydrocarbons are named, for example ‘triazan-1-yl’, tetrazan- 2-yl. The prefixes ‘triazano’ and ‘tetrazano’ previously used for ‘triazan-1-yl’ and ’tetrazan-1-yl’ and also...
read ...from ‘triazane’ and ‘tetraazane’ are named systematically as hydrocarbons are named,for example ‘triazan-1-yl’, ‘tetraazan- 2-yl’. The prefixes ‘triazano’ and ‘tetraazano’ previously used for ‘triazan-1-yl’ and ’tetraazan-1-yl’ and also...
Page xxxiii, 8 (c), line 2. [corrected 5 July 2018]
For ...semicarbazone, carbadiazone, and the...
read ...semicarbazone, and the...
Page xxxv, 11. (d), line 1. [corrected 3 October 2018]
For 2H-1-benzopyran
read 2H-1-benzopyran
Page xxxvi, 12 seniority (b), lines 3-5. [corrected 24 July 2019]
For ...(see P-67.1.2.6) and compounds such as R-NH-OH are named as N-derivatives of hydroxylamine, NH2-OH (see P-68.3.1.1.1) and not as N-substituted amines as in previous recommendations.
read ...(see P-67.1.2.6) and compounds such as R-NH-OH are named as N-derivatives of the senior amine and not as N-derivatives of hydroxylamine, NH2-OH (see P-62.4, P-68.3.1.1) as in previous recommendations.
Page xxxvii, entry 17 (c), line 4. [modified 3 March 2021]
For ...the latter is ‘1,3-dihydroxydisulfoxane-1,3-dione’; and the...
read ...the latter is ‘1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione’; and the...
Page xxxvii, 18. (b), lines 3-5. [corrected 16 January 2019]
For ... or cationic centers at the first atom in the order of classes (P-41) at the skeletal atom first cited in the seniority order of classes: N > P > As > Pb > Al > Ga...
read ...or cationic centers at the skeletal atom first cited in the seniority order of classes (P-41): N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga...
Page xxxviii, glossary, Attached component, line 3.
For ...in benzo[g]quinoline and...
read ...in benzo[g]quinoline and...
Page xxxix, glossary entry 6 on this page, lines 2/3. [corrected 6 May 2020]
For ...cyclohexyloxy (C6H11-CO–)
read ...cyclohexyloxy (C6H11-O–)
Page xli, glossary entry 12 (corrected to 13) on this page, lines 2-4. [corrected 3 March 2021]
For ... skeletal heteroatoms or to one carbon atom and one skeletal heteroatom, except for nitrogen and halogens, or to a heteroatom belonging to a ring or ring system, for example piperidin-2-one and 1-silylethan-1-one.
read ... skeletal heteroatoms or an acyclic compound, other than esters or acid anhydrides, having a carbonyl group linked to one carbon atom and one skeletal heteroatom, except for nitrogen and halogens, or an acyclic compound having a carbonyl group linked to a heteroatom belonging to a ring or ring system, for example piperidin-2-one, 1-silylethan-1-one and 1-(piperidin-1-yl)ethan-1-one.
Page 4, P-12.1, paragraph 2, line 7. [corrected 26 January 2022]
For ...substitutive names ‘vinylbenzene’, ‘phenylethene’, and ‘phenylethylene’. The name...
read ...substitutive name ‘vinylbenzene’. The name...
Page 4, P-12.1, example 6. [corrected 26 January 2022]
Delete phenylethene and phenylethylene
Page 6, P-12.1, Table 1.2, Formula 3' parent.
For phosphane (PIN)
read phosphane (preselected name)
Page 6, P-12.1, Table 1.2, Formula 6, name. [corrected 31 July 2019]
For 1-ethoxy-2-[2-(methoxyethoxy)ethoxy]ethane
read 1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane
Page 6, P-12.2, Table 1.2, Formula 7, name. [corrected 1 May 2019]
For 2-phenyloxirane
read phenyloxirane
Page 6, P-12.1, Table 1.2, Formula 8, parent.
For bornane (PIN)
read bornane
for bicycloheptane (PIN)
read bicyclo[2.2.1]heptane (PIN)
Page 6, P-12.1, table 1.2 example 9, name.
For 2-(1H-indol-1-yl)acetic acid
read (1H-indol-1-yl)acetic acid
Page 7, P-12.1, paragraph 4, line 8. [corrected 31 July 2019]
For 1-ethoxy-2-[2-(methoxyethoxy)ethoxy]ethane
read 1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane
Page 7, P-12.1, paragraph 4 line 17.
For 2-(1H-indol-1-yl)acetic acid
read (1H-indol-1-yl)acetic acid
Page 9, P-12.3, line 2.
For napthhalene
read naphthalene
Page 11, P-13.2.1.2, example 2. [corrected 30 January 2019]
For 4βH-4-carbayohimban
read (4βH)-4-carbayohimban
Page 12, P-13.2.2.2, example 2.
For hexane(dithioic) acid (PIN)
read hexane(dithioic acid) (PIN)
Page 12, P-13.2.2.2, example 3. [corrected 28 October 2016]
For 4-methaneselenoylbenzoic acid (PIN)
read 4-(methaneselenoyl)benzoic acid (PIN)
Page 13, P-13.2.2.3, line 3.
For ...furan, pyran (see Table 2.2)...
read ...pyran (see Table 2.2)...
Page 15, P-13.3.3.1, example 2. [corrected 21 August 2019]
For 2-phenyloxirane
read phenyloxirane
Page 19, P-13.4.3.2, example 2 on this page. [corrected 3 October 2018]
For 1,9-dinor(C60-Ih)[5,6]fullerene (PIN)
read 1,9-dinor(C60-Ih)[5,6]fullerene (PIN)
Page 21, P-13.6.1, example 2.
For nitrilotriacetic acid
read 2,2′,2′′-nitrilotriacetic acid
Page 22, P-13.6.2, example 2. [corrected 9 April 2025]
For dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read dimethyl butanedioylbis[oxy(2,1-phenylene)] dibutanedioate (PIN)
Page 24, P-13.8.1.2, example 1.
For (removal of the proline residue at positon 7 of oxytoxin
read (removal of the proline residue at positon 7 of oxytocin)
Page 25, P-13.8.2, example.
For 2,4,5-tri-O-methyl-D-mannitol
read 2,4,5-tri-O-methyl-D-mannose
for 3,6-anhydro-2,4,5-tri-O-methyl-D-mannitol
read 3,6-anhydro-2,4,5-tri-O-methyl-D-mannose
Page 26, P-14.1.2, Table 1.3, 2nd line last entry. [corrected 19 September 2018]
For Th
read Tl
Page 28, P-14.2.1.2, example 5.
For 23 tricos
read 23 tricosa
Page 30, P-14.3.4.1, line 3. [corrected 9 January 2019]
For ...hydrazides, nitriles, aldehydes...
read ...hydrazides, nitriles, esters, aldehydes...
Page 30, P-14.3.4.1, example 2. [corrected 1 May 2019]
For 2-chlorobutanedioic acid (PIN)
read chlorobutanedioic acid (PIN)
Page 31, P-14.3.4.2 (d). [corrected 9 April 2025]
For(d) in unsubstituted dinuclear and trinuclear...
read (d) in any dinuclear, unsubstituted trinuclear...
Page 31, P-14.3.4.2 (d), example 1.
For ethane
read ethene
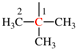
Page 31, P-14.3.4.3, example 1.
Replace the structure with:
CH3-NH-CO-NH2
Page 32, P-14.3.4.3, example 1 on this page. [corrected 19 September 2018]
For choropropanedioic acid (PIN)
read chloropropanedioic acid (PIN)
Page 32, P-14.3.4.4, example 2.
For (not 1-ethylidyne-2-ethylidynedisilane)
read (not 1-ethylidyne-2-methylidynedisilane)
Page 32, P-14.3.4.4, example 4. [corrected 5 July 2018]
For (not 1-ethylidene-2-methylidenetriphosphoxane)
read (not 1-ethylidene-5-methylidenetriphosphoxane)
Page 32, P-14.3.4.4, example 6.
For bromo(methyl)disulfane (PIN)
read (bromodisulfanyl)methane (PIN)
add [not bromo(methyl)disulfane see P-63.3.1; not 1-bromo-2-methyldisulfane]
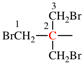
Page 32, P-14.3.4.4, example 9.
For ...i.e., 1-propylidene-2-ethylidenedisilan-1-yl)
read ...i.e., 2-ethylidene-1-propylidenedisilan-1-yl)
Page 32, P-14.3.4.4, example 10.
Replace structure with:

Page 32, P-14.3.4.4, after examples add. [corrected 9 April 2025]
For As an exception the locant is not omitted from propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant.
read As an exception the locant is not omitted from propa-1,2-diene, propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant.
Page 33, P-14.3.4.6, example 2. [modified 16 June 2021]
For (not 4-chorotrioxetane)
read (not 4-chloro-1,2,3-trioxetane)
Page 35, P-14.4 (b) lines 2/3. [corrected 28 May 2024]
For ....accommodate a substituent suffix in accordance with structural feature (d)
read ....accommodate a substituent suffix in accordance with structural feature (c)
Page 37, P-14.4 (f), example.
For (the locants set ‘2,4,5,8’ is lower than ‘2,4,7,8’)
read (the locants set ‘4,5,8’ is lower than ‘4,7,8’)
Page 37, P-14.4 (g), example 1.
For 4-methyl-5-nitrooctanedioic acid
read 4-methyl-5-nitrooctanedioic acid (PIN)
Page 38, P-14.4 (h), example 1. [corrected 5 July 2018]
Replace structure with:
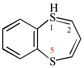
Page 38, P-14.4 (h), example 2.
Replace structure with:
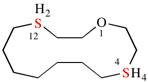
Page 38, P-14.4 (i), example 2.
For (2-14C,3,3-2H2)butane (PIN)
read (3-14C,2,2-2H2)butane (PIN)
for [not (3-14C,2,2-2H2)butane]
read [not (2-14C,3,3-2H2)butane]
Replace structure with:
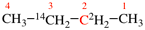
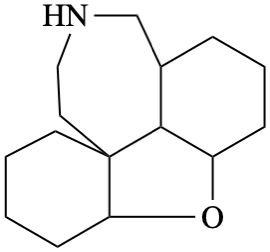
Page 39, P-14.4 (i), example 5 on this page.
For (2R)-[(1-131I)iodo]-3-iodo)propan-2-ol (PIN)
read (2R)-1-(131I)iodo-3-iodopropan-2-ol (PIN)
Page 40, P-14.4 (j), example 1 on this page.
Replace the structure with:
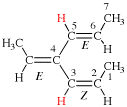
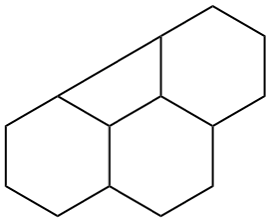
Page 41, P-14.4 (j), last example. [corrected 11 November 2020]
Replace the structure with:
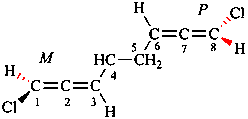
Page 42, P-14.5.1, example 4.
Replace structure with:
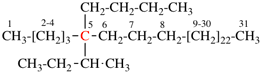
Page 44, P-14.5.4, example 1. [corrected 19 September 2018]
For 4-(2-methylbutyl)-N-(3-methylbutyl)benzeneamine
read 4-(2-methylbutyl)-N-(3-methylbutyl)benzenamine
Page 45, P-14.5.4, example 3 on this page.
Replace structure with:
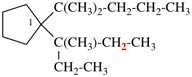
Page 46, P-14.6, example 2.
For 1-[4-(cyclohexylmethoxy)methyl]-4-{[4-(cyclohexylmethyl)cyclohexyl]methyl}cyclohexane (PIN)
read
1-[(cyclohexylmethoxy)methyl]-4-{[4-(cyclohexylmethyl)cyclohexyl]methyl}cyclohexane
for {not 1-({4-[(cyclohexylmethoxy)methyl]cyclohexyl}methyl)-4-(cyclohexylmethyl)cyclohexane [alphanumerical characters are identical; at the third character of the name, a square bracket is preferred to an open parenthesis}
read {not 1-({4-[(cyclohexylmethoxy)methyl]cyclohexyl}methyl)-4-(cyclohexylmethyl)cyclohexane; the alphabetic letters and the primary substituent locants are identical for both names; however, there is no locant for the first internal substituent in the PIN, but in the alternative name the locant for the first internal substituent is ‘4’ and no locant is preferred to ‘4’}
Page 46, P-14.6, example 3. [modified 28 April 2023]
For N-{2-[(acetyldimethylsilyl)methoxy]ethyl}-N′-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
read N1-(2-{[acetyldi(methyl)silyl]methoxy}ethyl)-N2-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
for N-[2-({2-[(acetyldimethylsilyl)methoxy]ethyl}amino)ethyl]-N′-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
read N1-{2-[(2-{[acetyldi(methyl)silyl]methoxy}ethyl)amino]ethyl}-N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
for ...nonalphanumerical ‘{’ is preferred to ‘(’]
read ...nonalphanumerical ‘{’ is preferred to ‘[’]
for N-(2-aminoethyl)-N′-(11,11-dimethyl-12-oxo-9-oxa-3,6-diaza-11-silatridecan-1-yl)ethane-1,2-diamine (PIN)
read 19-amino-3,3-dimethyl-5-oxa-8,11,14,17-tetraaza-3-silanonadecan-2-one (PIN)
Page 48, P-14.7.2, example 5 on this page. [corrected 10 October 2018]
Replace the structure with:
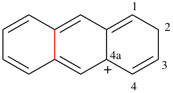
Page 49, P-14.8.1, line 9 on this page.
For ...by a double hyphen (--). The proportions...
read ...by a double hyphen (--) without spaces. The proportions...
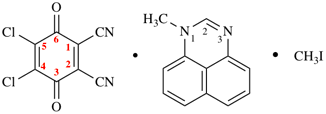
Page 49, P-14.8.1, example 1,
Replace structure with:

Page 50, P-14.8.1, example 1 on this page. [modified 11 December 2019]
For [(HOOC-CH2-NH)(CH2)2]2-N-CH2-COOH • HN[CH2-P(O)(OH)2]2 • H2N-(CH2)2-NH2
read (HOOC-CH2-NH-[CH2]2)2N-CH2-COOH • HN[CH2-P(O)(OH)2]2 • H2N-[CH2]2-NH2
for {[(carboxymethyl)azanediyl]bis(ethane-2,1-diylazanediyl)}diacetic acid—[iminobis(methylene)]bis(phosphonic acid—ethane-1,2-diamine (1/1/1)
read 2,2′-{[(carboxymethyl)azanediyl]bis(ethane-2,1-diylazanediyl)}diacetic acid—[azanediylbis(methylene)]bis(phosphonic acid—ethane-1,2-diamine (1/1/1)
for N,N-bis{2-[(carboxymethyl)amino]ethyl}glycine—[iminobis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
read N-{[(carboxymethyl)azanediyl]ethyl}-N,N′-(ethane-2,1-diyl)diglycine—[azanediylbis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
Page 50, P-14.8.1 example 2 on this page.
Replace structure with:
Page 51, P-14.8.1, example 2 on this page. [corrected 6 May 2020]
For ---2,2′-diylbis(diphenyl-λ5phosphanone) (1/1/1/1) (PIN)
read ---3,3′-diylbis(diphenyl-λ5phosphanone) (1/1/1/1) (PIN)
Page 51, P-14.8.1, example 3 on this page. [modified 18 September 2019]
For {piperazin-1-ium 115-[2-(2,4-dinitrophenyl)diazen-1-yl]-3,6,9,13,16,19-hexaoxa-...
read {piperazin-1-ium 115-[(2,4-dinitrophenyl)diazenyl]-3,6,9,13,16,19-hexaoxa-...
Page 53, P-14.8.2, last example.
For 4-[(4-aminomethyl)benzenesulfonyl]aniline—hydrogen chloride—water (1/1/1)
read 4-[4-(aminomethyl)benzene-1-sulfonyl]aniline—hydrogen chloride—water (1/1/1)
Page 54, P-15.0, line 17.
For oxydiaceticacid
read oxydiacetic acid
Page 55, P-15.0, line 1 on this page.
For α-aminoacids
read α-amino acids
Page 57, P-15.1.4, title.
For Position of the endings ‘‘ane’’, ‘ene’, and ‘yne’
read Position of the endings ‘ane’, ‘ene’, and ‘yne’
Page 59, P-15.1.7.1.3, text lines 2 and 4.
For (b)
read P-15.1.7.1.2
for (d)
read P-15.1.7.1.4
Page 61, P-15.1.7.2.3, line 2. [corrected 26 January 2022]
For ...For example, in a cyclic unsaturated alcohol having one substituent group...
read ...For example, in an unsaturated alcohol having one substituent group...
Page 64, P-15.1.8.2.1.2, example 2. [corrected 24 March 2021]
For N-phenylnitrous amide (PIN)
read phenylnitrous amide (PIN)
Page 64, P-15.1.8.2.1.2, example 2.. [corrected 10 June 2021]
For (not N-nitrosoaniline)
read N-nitrosoaniline
Page 65, P-15.1.8.3, example 2. [corrected 10 October 2018]
Replace the structure with:
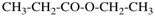
Page 67, P-15.2.1.2, example 3.
For ethyl methyl benzenedicarboxylate (PIN)
read ethyl methyl benzene-1,3-dicarboxylate (PIN)
Page 67, P-15.2.2, example 2.
For N,N-dimethylmethanamine oxide (PIN)
read N,N-dimethylmethanamine N-oxide (PIN)
for trimethylamine oxide
read trimethylamine N-oxide (traditional name, but cannot be substituted)
add trimethylazane N-oxide
Page 68, P-15.2.2, example 1 on this page. [corrected 24 July 2019]
For N-propylidenehydroxylamine (PIN)
read N-propylidenehydroxylamine
add N-hydroxypropan-1-imine (PIN)
Page 71, P-15.3.1.2.1.1, example 9.
Replace the structure with:
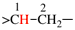
Page 72, P-15.3.1.2.1.2, example 5 on this page.
Replace the structure with:
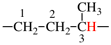
Page 73, P-15.3.1.2.2.1, example 1 on this page. [corrected 28 October 2016]
For [benzene-1,2,4-triyltris(oxy)] (preferred prefix)
read benzene-1,2,4-triyltris(oxy) (preferred prefix)
Page 73, P-15.3.1.2.2.2, example 2. [corrected 18 May 2023]
For ethane-1,2-diylidenebis(azanylylideneoxy)
read ethanediylidenebis(azanylylideneoxy)
Page 74, P-15.3.1.2.2.3, example 2.
For ethane-1,2- diylbis[azanylylidene(chloromethanylylidene)] (preferred prefix)
read ethane-1,2-diylbis[azanylylidene(chloromethanylylidene)] (preferred prefix) [removed space from name]
Page 74, P-15.3.1.2.2.4, example 3.
For peroxydi-4,1-phenylene (preferred prefix)
read peroxydi(4,1-phenylene) (preferred prefix)
for (not dioxydi-4,1-phenylene)
read [not dioxydi(4,1-phenylene)]
Page 75, P-15.3.1.2.2.4, example 2 on this page. [modified 31 July 2019]
For oxybis(1-chloroethane-2,1-diylphosphanylylideneethan-1-yl-2-ylidene)
read oxybis[(1-chloroethane-2,1-diyl)phosphanylylideneethan-1-yl-2-ylidene]
Replace the structure with:

Page 75, P-15.3.1.2.2.4, example 3 on this page. [corrected 31 July 2019]
Replace structure with:
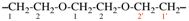
Page 75, P-15.3.1.2.2.4, example 4 on this page. [modified 24 March 2021]
For oxydipropan-1-yl-3-ylidene (preferred prefix)
read oxydi(propan-3-yl-1-ylidene) (preferred prefix)
replace structure with:
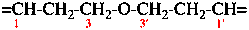
Page 75, P-15.3.1.2.2.4, example 1 on this page (example 4 of section). [corrected 9 April 2025]
For methylenebis(1,3,5-triazine-6,2,4-triyl) (preferred prefix)
read methylenedi(1,3,5-triazine-6,2,4-triyl) (preferred prefix)
Page 75, P-15.3.1.2.2.4, example 5 on this page. [modified 31 July 2019]
For hydrazinediylidenedipropan-1-yl-3-ylidene (preferred prefix;
read hydrazinediylidenedi(propan-1-yl-3-ylidene) (preferred prefix;
replace structure with:
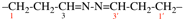
Page 77, P-15.3.2.1, example 1 on this page. [corrected 31 July 2019]
For 1,1′-[oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
read [oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
Page 77, P-15.3.2.1, example 6 on this page.
For 2,2′ -[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN)
read 2,2′-[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN) [Deleted space from name]
replace the structure with:

Page 78, P-15.3.2.1, example 4 on this page.
For [iminobis(methylene)]bis(phosphonic acid)
read [azanediylbis(methylene)]bis(phosphonic acid)
Page 78, P-15.3.2.1, example 5 on this page. [modified 25 August 2016]
For 1,1′,1′′,1′′′-[oxydi(pyridazine-4,3,5-triyl)]tetra(methan-1-ol) (PIN)
read [oxydi(pyridazine-4,3,5-triyl)]tetramethanol (PIN)
replace structure with:
Page 78, P-15.3.2.1, example 6 on this page. [corrected 31 July 2019]
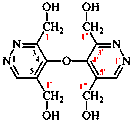
For 1,1′,1′′,1′′′-[oxydi(pyridazine-5,3,4-triyl)]tetra(methan-1-ol) (PIN)
read[oxydi(pyridazine-5,3,4-triyl)]tetramethanol (PIN)
delete [Note: The use of the locant ‘1’ here for methanol; see P-14.3.3]
replace structure with:

Page 79, P-15.3.2.1, example 3 on this page.
For 10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid).
read 10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid) (PIN)
Page 79, P-15.3.2.1, example 4 on this page. [corrected 31 July 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 80, P-15.3.2.1.1, example 1 on this page.
For 2,3′-oxydi(propanoic acid)
read 2,3′-oxydipropanoic acid
Page 80, P-15.3.2.2.1, example 1. [corrected 1 May 2019]
For N,N′-methylenedi(ethan-1-amine) (PIN)
read N,N′-methylenediethanamine (PIN)
Page 80, P-15.3.2.2.2, example 2.
Replace the structure with:
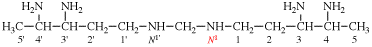
Page 81, P-15.3.2.3, example 1.
For 1,1′,1′′-(benzene-1,3,5-triyl)tris(silane) (PIN)
read (benzene-1,3,5-triyl)tris(silane) (PIN)
for (not benzene-1,3-5-triyltrisilane;...
read (not benzene-1,3,5-triyltrisilane;...
Page 81, P-15.3.2.3, example 2.
For 1,1′-[oxydi(tetradecane-14,1-diyl)]bis(silane) [preferred prefix; for...
read [oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN) [for...
Page 82, P-15.3.2.4.1, example 3 on this page. [corrected 6 May 2020]
Delete tetramethyldiazoxane (see P-21.2.3.1)
Page 82, P-15.3.2.4.1, example 4 on this page.
Delete 1,1′-(2-ethenyl-1,2,3-trimethyldisilazane-1,3-diyl)bis(ethan-1-one)
Page 82, P-15.3.2.4.1, last example on this page.
For 1,1′,1′′′-( benzene-1,3-5-triyl)tris(trimethylsilane) (PIN)
read (benzene-1,3,5-triyl)tris(trimethylsilane) (PIN) [delete space from name, and change hyphen to comma]
Page 83, P-15.3.2.4.2, example 1.
For [not 3-[(5-chloro-5-cyanophenyl)methyl]benzonitrile; the PIN name has the greater number of substituents; see P-45.1.1]
read [not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile; the PIN has the greater number of substituents; see P-45.2.1]
Page 83, P-15.3.2.4.2, example 2.
For 2-chloro-4-[2-(5-chloro-2-hydroxyphenyl)ethyl]phenol (PIN)
read [not 2-chloro-5-[2-(5-chloro-2-hydroxyphenyl)ethyl]phenol; the locant set for the substituents in the PIN, ‘2,4’, is lower than ‘2,5’; see P-14.4 (f); P-45.2.2]
for [not 4-chloro-2-[2-(4-chloro-3-hydroxyphenyl)ethyl]phenol; the locant set for the substituents in order of their occurence in the PIN, ‘2,4’, is lower than ‘4,2’; see P-45.2.2]
read 4-chloro-2-[2-(4-chloro-3-hydroxyphenyl)ethyl]phenol (PIN)
Page 84, P-15.3.2.4.2, example 1 on this page. [corrected 30 January 2019]
For [(2-chlorosilyl)ethyl]silane
read [2-(chlorosilyl)ethyl]silane
Page 84, P-15.3.2.4.2, example 2 on this page.
For 1,1,1-trimethyl-2-(disilanyldisulfanyl)disilane (PIN)
read 2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
for [not [2-(trimethyldisilanyl)disulfanyl]disilane; the parent disilane in the PIN has more substituents; see P-45.1.1]
read [not [2-(2,2,2-trimethyldisilan-1-yl)disulfanyl]disilane; the parent disilane in the PIN has more substituents; see P-45.2.1]
Page 84, P-15.3.2.4.2, example 3 on this page.
For 2-chloro-4-(5-chloropyridin-2-yloxy)pyridine (PIN)
read 2-chloro-4-[(5-chloropyridin-2-yl)oxy]pyridine (PIN)
Page 84, P-15.3.2.4.3, example 1.
For 3-bromo-1-[(3-chlorophenyl)methyl]benzene (PIN)
read 1-bromo-3-[(3-chlorophenyl)methyl]benzene (PIN)
Page 85, P-15.3.2.4.3, last example.
For 5-bromo-2-(2-carboxy-4-fluorophenoxy)benzoic acid (PIN)
read 2-(4-bromo-2-carboxyphenoxy)-5-fluorobenzoic acid (PIN)
for [not 2-(4-bromo-2-carboxyphenoxy)-5-fluorobenzoic acid; ‘bromocarboxyfluoro’ is preferred alphanumerically to ‘bromocarboxyphenoxy’; see P-45.5]
read [not 5-bromo-2-(2-carboxy-4-fluorophenoxy)benzoic acid; ‘2,5’ is preferred to ‘5,2’; see P-45.2.3]
Page 85, P-15.3.3.1, Example 1.
For 1,1′-(propane-1,2-diyl)bis(trimethylsilane) (PIN)
read (propane-1,2-diyl)bis(trimethylsilane) (PIN)
Page 85, P-15.3.3.1, example 2.
For [not 1-bromo-4-[3-(4-bromophenyl)prop-3-en-1-yl]benzene;...
read [not 1-bromo-4-[3-(4-bromophenyl)prop-2-en-1-yl]benzene;...
Page 86, P-15.3.3.2.1, example 2. [corrected 30 January 2019]
For {(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methanediyl}dibenzene
read 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene
for [not ({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene; ...
read [not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene; ...
Page 86, P-15.3.3.2.2, example.
For 1,1′-[carbonylbis(diazene-2,1-diyl)]dibenzene
read 1,1′-[carbonylbis(diazenediyl)]dibenzene
Page 87, P-15.3.4.1.1, example (1). [modified 30 January 2019]
For 1-[(disilanylmethyl)peroxy]disilane (PIN)
read [(disilanylmethyl)peroxy]disilane (PIN)
for [not (disilanylperoxy)methyl]disilane;
read [not [(disilanylperoxy)methyl]disilane;
Page 87, P-15.3.4.1.1, example (2).
For 4-{[(4-hydroxyphenyl)amino]methyl}phenol (PIN)
read 4-[(4-hydroxyanilino)methyl]phenol (PIN)
for ‘hydroxyphenylaminomethyl’ precedes ‘hydroxyphenylmethylamino’ ...
read ‘hydroxyanilinomethyl’ precedes ‘hydroxyphenylmethylamino’ ...
Page 87, P-15.3.4.1.1 example (3). [modified 6 May 2020]
For 1-{[2-(disilan-1-yloxy)ethyl]sulfanyl}disilane
read {[2-(disilanyloxy)ethyl]sulfanyl}disilane
for [not 1-{[2-(disilan-1-ylsulfanyl)ethoxy}disilane;
read [not [2-(disilanylsulfanyl)ethoxy]disilane;
Page 88, P-15.3.4.2, example.
For 2-(ethylsulfanyl)-1,1-bis(propylsulfanyl)ethene (PIN...
read 1-{[2-(ethylsulfanyl)-1-(propylsulfanyl)ethen-1-yl]sulfanyl}propane (PIN...
Page 94, P-15.5.2, line 12. [corrected 6 May 2020]
For ...and 1-amido-2-imidodiphosphonic chloride (see P-67.2.3).
read ...and 3-amido-2-imidodiphosphonic chloride (see P-67.2.3).
Page 96, P-15.5.3.2, Table 1.6, line 1 on this page.
For isothiocyantido
read isothiocyanatido
Page 100, P-15.6.1.4, example 3. [corrected 25 August 2016]
For pyridine-2,3-bis(decanoic acid)
read pyridine-2,3-di(decanoic acid)
for 10,10′-(pyridine-2,3-diyl)bis(decanoic acid) (PIN)
read 10,10′-(pyridine-2,3-diyl)di(decanoic acid) (PIN)
Replace the structure with:
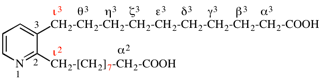
Page 102, P-15.6.2, last example. [modified 30 January 2019]
For β,5-dihydroxybenzene-1,3-diethanol
read β1,5-dihydroxybenzene-1,3-diethanol
for [5-hydroxy-3-(2-hydroxyethyl)phenyl]ethane-1,2-diol
read 1-[3-hydroxy-5-(2-hydroxyethyl)phenyl]ethane-1,2-diol
Page 104, P-15.6.3, example at top of page.
For cyclohexaneheptanal
read benzeneheptanal
Page 105, P-16.2.1 (b), example 1. [corrected 19 September 2018]
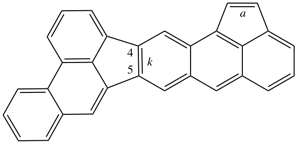
For dibenzo[c,g]anthracene [PIN, P-25.3.4.2.1 (c)]
read dibenzo[c,g]phenanthrene [PIN, P-25.3.4.2.1 (c)]
Page 106, P-16.2.5.
For P-16.2.5
read P-16.2.4.5
Page 106, P-16.2.4.5 (was P-16.2.5), example.
For carbon monoxide — methylborane (PIN...
read carbon monoxide—methylborane (PIN... [Deleted spaces from name]
Page 106, P-16.2.6.
For P-16.2.6
read P-16.2.5
Page 106, P-16.2.5 (was P-16.2.6) (b), example 2 on this page.
For 2-(carbonochloridothioyl)benzoyl cyanide
read 2-(carbonocyanidothioyl)benzoyl chloride
Page 107, P-16.2.5 (was P-16.2.6) (b), example 2 on this page. [corrected 19 September 2018]
For 1-(ethanesulfonyl)butane (PIN)
read 1-(ethanesulfinyl)butane (PIN)
Page 107, P-16.2.5 (was P-16.2.6) (c), example 2. [corrected 24 July 2019]
For (pentan-2-ylidene)hydroxylamine (PIN)
read (pentan-2-ylidene)hydroxylamine
add N-hydroxypentan-2-imine (PIN)
Page 107, P-16.2.5 (was P-16.2.6) (d), example 2.
For trimethyl-λ5-phosphanone (PIN, P-68.2.3.2.1, P-74.2.1.4)
read trimethyl-λ5-phosphanone (PIN, P-68.3.2.3.1, P-74.2.1.4)
Page 107, P-16.3.2 (a), line 3. [corrected 31 July 2019]
For ...ethyl or t-butyl...
read ...ethyl or tert-butyl...
Page 109, P-16.3.4 (d), example 3. [corrected 11 December 2019]
For tetra(decanoic acid) (preferred suffix, defines four...
read tetra(decanoic acid) (defines four...
Page 110, P-16.3.5 (b), example 1. [modified 11 December 2019]
For bis(thioic) acid [preferred suffix, describes two – C(O/S)H suffixes, see P-65.1.5.1; whereas dithioic acid describes –CSSH suffix, see P-65.1.5.1]
read bis(thioic acid) (multiple preferred suffix, describes two –C(O/S)H suffixes, see P-65.1.5.1) [delete space in structure]
Page 110, P-16.3.5 (b), example 2. [modified 11 December 2019]
For bis(dithioic) acid (preselected suffix, see P-65.1.5.1) not didithioic acid
read bis(dithioic acid) (multiple preferred suffix, describes two –CSSH suffixes, see P-65.1.5.1) not didithioic acid
Page 112, P-16.3.6(b), example 1 on this page. [corrected 1 May 2019]
For bis(azo) (preferred prefix, defines two...
read bis(azo) (defines two...
for 3,3′-[methylenebis(azo)]dipropanoic acid (PIN)
read 3,3′-[methylenebis(azo)]dipropanoic acid
Page 114, P-16.5.1.1, example 3. [corrected 28 October 2016]
For not 1,3,5-tris(decy)lcyclohexane
read not 1,3,5-tris(decyl)cyclohexane
Page 114, P-16.5.1.1, last example. [corrected 30 January 2019]
Delete 1,4-phenylenedi(tridecane).
Page 115, P-16.5.1.2, example 3. [corrected 8 July 2017)
For 1-(naphthalen-2-yl)-2-phenyldiazene (PIN...
read (naphthalen-2-yl)(phenyl)diazene (PIN...
Page 115, P-16.5.1.3, lines 1-4. [modified 6 December 2023]
For P-16.5.1.3 Parentheses are placed around prefixes denoting simple substituent groups in front of parent hydrides when no locants are necessary in order to avoid any misunderstanding that the substituent group is modified by a preceding one. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses. Parentheses are placed also around prefixes denoting simple substituent groups qualified by locants. A minimum of parentheses must be used. Enclosing marks are never used around the names of the first cited simple substituent group.
read P-16.5.1.3 Parentheses for substituents of parent structures.
P-16.5.1.3.1 For mononuclear parent hydrides with two or more substituents the first cited substituent never has enclosing marks unless it is includes a locant. The second and further substituents are each enclosed with parentheses even for simple substituents. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses.
Page 115, P-16.5.1.3 (now P-16.5.1.3.1), example 1.. [corrected 12 May 2021]
For butyl(ethyl)methyl(propyl)silane (PIN)
read butyl(ethyl)(methyl)(propyl)silane (PIN)
Page 115, P-16.5.1.3 (now P-16.5.1.3.1), example 3.. [corrected 12 May 2021]
For ethyl(methyl)propylphosphane (PIN)
read ethyl(methyl)(propyl)phosphane (PIN)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 1 on this page. [corrected 12 May 2021]
For ethyl(isopropyl)methylphosphane
read ethyl(isopropyl)(methyl)phosphane
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 2 on this page. [corrected 8 July 2017)
For (chloromethyl)methylsilane (PIN; P-68.2.6.1)
read (chloromethyl)(methyl)silane (PIN; P-68.2.6.1)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 4 on this page. [corrected 20 February 2019]
For ethyl(dimethyl)phosphane (PIN)
read ethyldi(methyl)phosphane (PIN)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 5 on this page. [corrected 5 July 2018]
For ethyl[di(propan-2-yl)]propylsilane (PIN)
read ethyldi(propan-2-yl)silane (PIN)
add ethyldiisopropylsilane
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), after example 5.. [corrected 12 May 2021]
Add ethyl(methyl)(propyl)silanecarboxylic acid

methyl(phenyl)phosphinic acid

cyclopropyl(phenyl)methanol

Page 116, P-16.5.1.3 (now P-16.5.1.3.1), after P-16.5.1.3.1. [corrected 12 May2021]
Add P-16.5.1.3.2 Simple substituents of a parent where only one possible position can be substituted do not require locants. Enclosing marks are used with second and subsequent simple substituents (see P-16.5.1.3.1). In some cases, such as acetic acid, alkyl substituents are not allowed as the resulting compound requires a different parent structure. Locants are required for related compounds where additional substitutable positions are available, for example acetamide.
bromo(nitro)(phenyl)acetic acid

diethyl ethyl(methyl)propanedioate

bromo(chloro)acetic acid

cyclopropyl(fluoro)acetonitrile

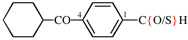
Page 116, P-16.5.1.4, example 1. [modified 7 November 2018]
For 4-(cyclohexanecarbonyl)benzenecarbothioic acid (PIN)
read 4-(cyclohexanecarbonyl)benzene-1-carbothioic acid (PIN)
replace the structure with:
Page 117, P-16.5.1.8, example.
For 4-ethanethioylbenzoic acid (PIN...
read 4-(ethanethioyl)benzoic acid (PIN...
Page 117, P-16.5.1.9, example 1. [modified 16 January 2019]
For {benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)}-5-carboxylic acid (PIN; P-65.1.2.2.2)
read [benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid (PIN; P-65.1.2.2.2)
Page 118, P-16.5.1.11, example 3.
Replace structure with:
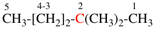
Page 119, P-16.5.3.1, example.
Replace the structure with:
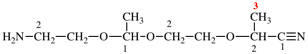
Page 119-120, P-16.5.4, Fig 1.3.
Replace the structure and text with:

(a) = 4′-cyano[1,1′-biphenyl]-4-yl
(b) = (4′-cyano[1,1′-biphenyl]-4-yl)oxy
(c) = 5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl
(d) = {5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy
(e) = 3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl
(f) = 4,4′-[3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl]dibenzoic acid (PIN)
Page 120, P-16.5.4, example on this page. [corrected 28 October 2016]
For [N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-ethane-1,2-diyldiglycine
read N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
Page 120, P-16.5.4.1. [corrected 6 December 2023]
Replace section (retain box at end)
P-16.5.4.1 The presence of square brackets and/or parentheses that are an integral part of the name of a parent structure may affect the nesting order as described below.
P-16.5.4.1.1 In determining the nesting order in a name the parentheses of added indicated hydrogen are ignored.
Example:
Examples:
Examples:
Example:
Explanation: The order of insertion of stereochemistry for 2-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid is as shown below.
Stage 1 insert stereochemistry at the inner most position:2-{2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl requires square brackets to surround it hence subsequent enclosing marks need to be adjusted.
Stage 2 insert stereochemistry at the next position:
2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl has a parenthesis on the left and brace on the right so to make clear it requires square brackets around it.
Stage 3 insert stereochemistry at the third position:
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
Page 121, P-16.6.1, example 2. [corrected 23 December 2020]
Delete this example.
Page 121, P-16.6.2, example 1.
For N,N-diethylethan-1-amine (PIN)
read N,N-diethylethanamine (PIN)
Page 122, P-16.7.1 (a), line 2.
For ...when followed by a suffix or 'en' ending beginning with 'a', 'e'....
read ...when followed by a suffix or ending beginning with 'a', 'e'....
Page 123, P-16.7.2 (d), example 2. [modified 31 July 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 124, P-16.8.2, example 1. [corrected 9 January 2019]
For (not but-1,2-diene)
read (not but-1,3-diene)
Page 124, P-16.9.1, example 1.
For N′-acetyl-N′-ethyl-N-methylpropanehydrazide
read N′-acetyl-N′-ethyl-N-methylbutanehydrazide
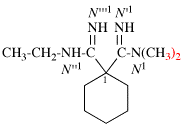
Page 126, P-16.9.3, example 2.
For N′′1-ethyl-N1,N1,-dimethylcyclohexane-1,1-dicarboximidamide (PIN, P-66.4.1.4.2)
read N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN, P-66.4.1.4.2)
for N′′′-ethyl-N,N,-dimethylcyclohexane-1,1-dicarboximidamide
read N′′-ethyl-N,N-dimethylcyclohexane-1,1-dicarboximidamide
Replace structure with:
Page 127, P-16.9.3, example 5 on this page.
For N1,N′1-methylenedi(pentane-1,3,4-triamine) (PIN, P-62.25.2)
read N1,N1′-methylenedi(pentane-1,3,4-triamine) (PIN, P-62.25.2)
Page 127, P-16.9.3, last example on this page.
For N1-ethyl- N1′ ,N1′,N3-trimethylpropane-1,1,3-tricarboxamide (PIN, P-66.1.1.3.1.2)
read
N′1-ethyl-N1,N1,N3-trimethylpropane-1,1,3-tricarboxamide (PIN, P-66.1.1.3.1.2) [delete spaces from name]
Replace structure with:
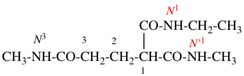
Page 128, P-16.9.3, example 1 on this page. [corrected 19 September 2018]
For N1, N1, N′3-triethyl-N′1, N3, N3-trimethylnapthalene-1,3-dicarboximidamide (PIN, P-66.4.1.4.2)
read N1,N1,N′3-triethyl-N′1,N3,N3-trimethylnaphthalene-1,3-dicarboximidamide (PIN, P-66.4.1.4.2) [deleted spaces from name]
Page 128, P-16.9.4 (b), example 2 on this page.
For 1H,1′H,1′′H,3′H-2,2′:7,2′′-dispiroter[naphthalene]
read 1H,1′H,1′′H,3′H-2,2′:7′,2′′-dispiroter[naphthalene]
Page 129, P-16.9.5 (a), example 1. [corrected 31 July 2019]
For pyrido[1′′,2′′:1,2′]imidazo[4′,5′:5,6]pyrazino[2,3-b]phenazine (PIN,
read pyrido[1′′,2′′:1′,2′]imidazo[4′,5′:5,6]pyrazino[2,3-b]phenazine (PIN,
Page 129, P-16.9.5 (a), example 2.
For difuro[3,2-b;2′,3′-e]pyridine (PIN,
read difuro[3,2-b:2′,3′-e]pyridine (PIN,
Page 129, add a new section:
P-16.9.6. Multiple primes
Long strings of primes may be needed with ring assemblies and polyspiro compound. It is difficult to count long streams of primes. For example, how many primes appear in this string: ′′′′′′′′ ? It is eight. In these recommendations, to simplify the counting process, long strings of primes are broken into groups of three as: ′′′ ′′′ ′′, which has the same number of primes as the string above. This is a change from the publication on spiro compounds (ref. 8) in which multiple primes were separated by a space after every four primes.
Page 130, P-20, lines 9/10. [modified 28.8.2019]
For ...for example, spiro[1,3-dioxolane-2,1′-[1H]indene], and there...
read ...for example, 1′H-spiro[[1,3]dioxolane-2,1′-indene], and there...
Page 131, P-21.1.1.1, line 6. [corrected 27 May 2020]
For ...tellane for H2Te, polonane for H2Po, and...
read ...tellane for H2Te, polane for H2Po, and...
Page 131, P-21.1.1.1, line 8.
For ...polane and bismane...
read ...polonane and bismane...
Page 132, P-21.1.1.1, example 4.
Change structure to:
Page 135, P-21.2.3.1, example 5.
For N-silylsilan-1-amine
read N-silylsilanamine
Page 135, P-21.2.3.1, example 6. [corrected 11 November 2020]
Delete this example
Page 137, P-21.2.4.2, example 2. [corrected 28 April 2023]
For 2,5λ4,8,11-tetrathiadodecane
read 2,5λ4,8λ6,11-tetrathiadodecane
replace the structure with:
Page 140, P-22.2.1, Table 2.2, structure 2.
Replace the structure with:
Page 140, P-22.2.1, Table 2.2, structure 4.
For 1, 3-selenazole (PIN)
read 1,2-selenazole (PIN) [delete space in name]
for 1,3-tellurazole (PIN)
read 1,2-tellurazole (PIN)
Page 142, P-22.2.1, Table 2.3 structure 1.
For 1,3-thioxazolidine (PIN)
read 1,3-thiazolidine (PIN)
for 1,3-selenoxazolidine (PIN)
read 1,3-selenazolidine (PIN)
for 1,3-telluroxazolidine (PIN)
read 1,3-tellurazolidine (PIN)
Page 142, P-22.2.1, Table 2.3 structure 2.
For isothioxazolidine (S instead of O)
read isothiazolidine (S instead of O)
for 1,2-thioxazolidine (PIN)
read 1,2-thiazolidine (PIN)
for isoselenoxazolidine (Se instead of O)
read isoselenazolidine (Se instead of O)
for 1,2-selenoxazolidine (PIN)
read 1,2-selenazolidine (PIN)
for isotelluroxazolidine (Te instead of O)
read isotellurazolidine (Te instead of O)
for 1,2-telluroxazolidine (PIN)
read 1,2-tellurazolidine (PIN)
Page 146, P-22.2.2.1.5.1, lines 1/2. [corrected 9 January 2019]
For ...rings containing only nitrogen; otherwise...
read ...rings only containing nitrogen heterotoms; otherwise...
Page 149, P-22.2.3.2.1, example 2.
For 1H-1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
read 1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
Page 150, P-22.2.4, line 8.
For (see P-22.2.4)
read (see P-22.2.3)
Page 151, P-22.2.5, example 1. [modified 11 November 2020]
Delete pentazolane
Page 152, P-22.2.6, last line.
For ...heteromonocyclic hydrides, see P-52.1.7.
read ...heteromonocyclic hydrides, see P-52.1.5.2.
Page 152, P-22.2.6, example 2. [corrected 28 October 2016]
For 3,5,2,4,6-triphosphatriborinane
read 1,3,5,2,4,6-triphosphatriborinane
Page 160, P-23.2.6.2.3, right-hand structure of example 2.
For tetracyclo[5.3.2.12,4.06.13]tridecane
read tetracyclo[5.3.2.12,4.06,13]tridecane
Page 161, P-23.2.6.2.4, example 1.
Replace the structures with:
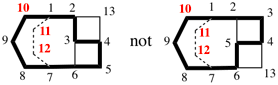
Page 161, P-23.2.6.2.4, example 2, left version. [modified 8 May 2019]
For tricyclo[5.5.1.03,11]tridecane (PIN)
read tricyclo[5.5.1.03,11]tridecane
replace the structure with:
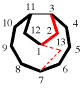
Page 162, P-23.2.6.2.5, example 1, right structure. [corrected 30 January 2019]
Replace structure with:
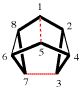
Page 162, P-23.2.6.2.5, example 2, left-hand structure.
Replace the structure with:
Page 162, P-23.2.6.3, line 5.
For ... atom of the main bridge, then ...
read ... atom of the main ring, then ...
Page 167, P-23.5.1, example 1 on this page.
Replace the structure with:
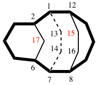
Page 167, P-23.5.2, example 2.
For (b) 2,4,6,8,9-pentaaza-1,3,5,7,10-pentasilatricyclo[3.1.1.12,4]decane
read (b) 2,4,6,8,9-pentaaza-1,3,5,7,10-pentasilatricyclo[3.3.1.12,4]decane
replace the structure with:
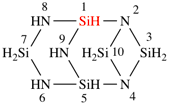
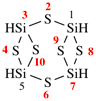
Page 168, P-23.5.3, line 8.
For Si2nH3nN3n
read Si2nH5nN3n
Page 168, P-23.6.2, line 4.
For phosphorus
read arsenic
Page 169, P-23.7, Table 2.6 structure 4.
Replace structure with:
Page 175, P-24.2.3.1, example 1 on this page. [corrected 4 September 2019]
For tetraspiro[2.2.26.2.2.214.211.23]icosane (PIN)
read tetraspiro[2.2.26.2.2.214.211.23]icosane
Page 180, P-24.3.3, example 2.
For 4H-2,4′-spirobi[[1,3]dioxolo[4,5-c]pyran] (PIN)
read 2′H,4H-2,4′-spirobi[[1,3]dioxolo[4,5-c]pyran] (PIN)
Page 180, P-24.3.3, last example.
For 2,4′spirobi[1]benzopyran (PIN)
read 2,4′-spirobi[[1]benzopyran] (PIN)
Page 182, P-24.4, title.
For MONOSPIRO RING SYSTEMS ...
read DISPIRO RING SYSTEMS ...
Page 182, P-24.4.1, example 2.
Change structure to:
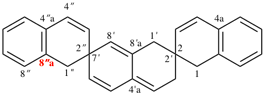
Page 184, P-24.4.3, example 1.
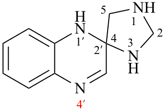
For correct 1′H,2H,3′′H,4a′H-3,7′:2′,7′′-dispiroter[quinoline]
read correct 1′H,2H,3′′H,4′aH-3,7′:2′,7′′-dispiroter[quinoline]
change correct structure to:
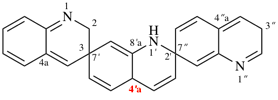
and
For incorrect 1′H, 2′′H,3H,4a′H-7,2′:7′,3′′-dispiroter[quinoline]
read incorrect 1′H,2′′H,3H,4′aH-7,2′:7′,3′′-dispiroter[quinoline] [delete space from name]
change incorrect structure to:
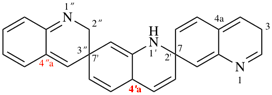
Page 184, P-24.4.3, example 1, Explanation. [corrected 23 January 2019]
For The locant set ‘1′,2,3′′...’ in the correct name, the PIN, is lower than the locant set ‘1′,2′′,3′′...’ in the incorrect name
read The locant set ‘2′,3,7′,7′′...’ in the correct name, the PIN, is lower than the locant set ‘2′,3′′,7,7′...’ in the incorrect name.
Page 184, P-24.4.3, last example.
For 6,6′,6′′,8,8′,8′′- hexaoxa[2,7′:2′,7′′]dispiroter[bicyclo[3.2.1]octane (PIN)
read 6,6′,6′′,8,8′,8′′-hexaoxa-2,7′:2′,7′′-dispiroter[bicyclo[3.2.1]octane] (PIN) [delete space in name]
Page 185, P-24.5.1, example 3. . (corrected 23 October 2019)
Replace the structure with:
Page 186, P-25.5.3, line 4/8. [corrected 8 May 2019]
For Locants present in bicyclic fused benzo ring components are cited directly in front of the component and name when they correspond to the numbering of the spiro ring system; when they do not correspond to those of the spiro ring system, they are placed in brackets (see third and fourth examples below).
read All locants present in a bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second component, see third and fourth examples below).
Page 186, P-24.5.2, example 1. [corrected 4 September 2019]
For [not 2-thiaspiro[bicyclo[2.2.2]octane-3,9′- fluorene] (II)]
read [not 2-thiaspiro[bicyclo[2.2.2]octane-3,9′-fluorene] (II)] [delete space in name]
Page 187, P-24.5.3, example 4 on this page. [modified 4 September 2019]
For (3,1-benzoxazine before 2,3,-benzoxazine)
read (3,1-benzoxazine before 2,3-benzoxazine)
Replace the structure with:
Page 188, P-24.6, line 7/8. [corrected 8 May 2019]
For ... and so on; accordingly, locants required by the names of the second and later ring components are enclosed in square brackets.
read ... and so on. In a complete name, all locants present in a bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second and subsequent components, see fourth example below).
Page 188, P-24.5.4, example 1. [corrected 4 September 2019]
For [bicyclo[2.2.2]octane is cited before bicyclo[3.2.1]octane
read [bicyclo[2.2.2]octane is cited before bicyclo[3.2.1]octane]
Page 189, P-24.6, example 3.
For 7′-azadispiro[fluorene-9,2′-bicyclo[2.2.1]heptane-5′,1′′-indene]
read
7′-azadispiro[fluorene-9,2′-bicyclo[2.2.1]heptane-5′,1′′-indene] (PIN)
Page 189, P-24.6, example 4.
For 3,6-dioxadispiro[bicyclo[2.2.1]heptane-2,2′-[1,4]dioxane-5′,2′′-pyran (PIN)
read 3,6-dioxadispiro[bicyclo[2.2.1]heptane-2,2′-[1,4]dioxane-5′,2′′-pyran] (PIN)
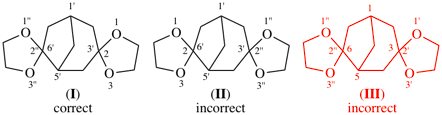
Page 190, P-24.6.1, example. [modified 27 May 2020]
For dispiro[[1,3]dioxolane-2,3′-bicyclo[3.2.1]octane-6′,2′′-[1,3]dioxolane] (I)
read dispiro[[1,3]dioxolane-2,3′-bicyclo[3.2.1]octane-6′,2′′-[1,3]dioxolane] (I) (PIN)
For dispiro[bicyclo[3.2.1]octane-2,3′:6′,2′′-bis([1,3]dioxolane) (PIN, see P-24.7.1)
read [not dispiro[bicyclo[3.2.1]octane-3,2′:6,2′′-bis([1,3]dioxolane) (III) (see P-24.4.2)]
Replace structures with:
Page 190, P-24.7.1, example. [corrected 8 May 2019]
read trispiro[1,3,5-trithiane-2,2′:4,2′′:6,2′′′-tris(bicyclo[2.2.1]heptane)] (PIN)
read trispiro[[1,3,5]trithiane-2,2′:4,2′′:6,2′′′-tris(bicyclo[2.2.1]heptane)] (PIN)
Page 192, P-24.7.2, example (3). [modified 27 May 2020]
Replace the structures with:
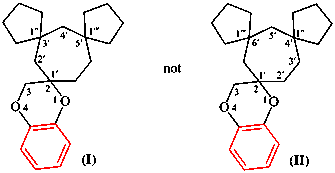
for trispiro[bis(cyclopentane)-1,1′:1,4′-cycloheptane-6′,2′′′-[1,4]-dioxolane] (I) (PIN)
read 3′′′H-trispiro[bis(cyclopentane)-1,1′:4′,1′′-cycloheptane-6′,2′′′-[1,4]benzodioxine] (I) (PIN)
for (not trispiro[bis(cyclopentane)-1,1′:1,5′-cycloheptane-3′,2′′′-[1,4]-dioxolane] (II)
read [not 3′′′H-trispiro[bis(cyclopentane)-1,1′:5′,1′′-cycloheptane-3′,2′′′-[1,4]benzodioxine] (II)]
Page 192, P-24.7.2, example (3), Explanation line 4.
For ...where 4′ is lower than 5′)
read ...where 4′ is lower than 5′.
Page 192, P-24.7.2, example (3). [corrected 11 November 2020]
Replace the structures with:

Replace the text by:
Page 192, P-24.7.2, example (4).
For trispiro[cyclopentane-1,1′-cyclohexane-3′,2′′- imidazole-5′,1′′′-indene
read trispiro[cyclopentane-1,1′-cyclohexane-3′,2′′-imidazole-5′,1′′′-indene [Deleted space from name]
Page 193, P-24.7.3, example. [modified 27 May 2020]
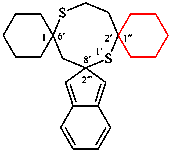
Replace the structure of (I) with:
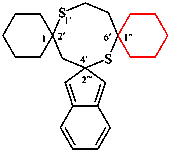
replace the structure of (II) with:
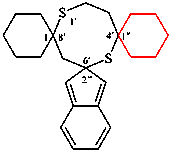
replace the structure of (III) with:
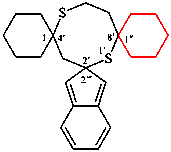
replace the structure of (IV) with:
for trispiro[cyclohexane-1,2′-[1,5]dithiocane-6′,1′′-cyclopentane-4′,2′′′-indene] (I) (PIN)
read trispiro[bis(cyclohexane)-1,2′:6′,1′′-[1,5]dithiocane-4′,2′′′-indene] (I) (PIN)
for trispiro[cyclohexane-1,8′-[1,5]dithiocane-4′,1′′-cyclopentane-6′,2′′′-indene] (II)
read [not trispiro[bis(cyclohexane)-1,8′:4′,1′′-[1,5]dithiocane-6′,2′′′-indene] (II)
for trispiro[cyclohexane-1,4′-[1,5]dithiocane-8′,1′′-cyclopentane-4′,2′′′-indene] (I)
read nor trispiro[bis(cyclohexane)-1,4′:8′,1′′-[1,5]dithiocane-2′,2′′′-indene] (III);
for trispiro[cyclohexane-1,6′-[1,5]dithiocane-2′,1′′-cyclopentane-8′,2′′′-indene] (I)
read nor trispiro[bis(cyclohexane)-1,6′:2′,1′′-[1,5]dithiocane-8′,2′′′-indene] (IV)]
Page 194, P-24.7.4.1, example 1. [modified 27 May 2020]
Delete the correction to the first name
Page 194, P-24.7.4.1, example 2. [modified 27 May 2020]
For 1λ5,3λ5,5λ5,7λ5-tetraspiro[tetraspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphaadamantane-1,2′:3,2′′:5,2′′′:7,2′′′ ′-tetrakis([1,3,2]oxathiaphosphetane)]-4′,7′′′ ′′, 4′′,7′′′ ′′′,:4′′′,7′′′ ′′′ ′,4′′′ ′,7′′′ ′′′ ′′-tetrakis(pyrano[2,3-c]acridine)} [PIN, see SP-7.4(b)]
read 1λ5,3λ5,5λ5,7λ5-tetraspiro{tetraspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphaadamantane-1,2′:3,2′′:5,2′′′:7,2′′′ ′-tetrakis([1,3,2]oxathiaphosphetane)]-4′,7′′′ ′′:4′′,7′′′ ′′′: 4′′′,7′′′ ′′′ ′:4′′′ ′,7′′′ ′′′ ′′-tetrakis(pyrano[2,3-c]acridine)} [PIN, see SP-6.4(b), in ref 8]
for
octaspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane-1,2′λ5:3,2′′λ5:5,2′′′λ5:7,2′′′′λ5-tetrakis[1,3,2]oxathiaphosphetane- 4′,7′′′ ′′:4′′,7′′′ ′′′:=4′′′,7′′′ ′′′ ′:4′′′,7′′′ ′′′ ′′-tetrakis[7H]pyrano[2,3-c]acridine] (the CAS index name)
read octaspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane-1,2′λ5:3,2′′λ5:5,2′′′λ5:7,2′′′′λ5-tetrakis[1,3,2]oxathiaphosphetane-4′,7′′′′′:4′′,7′′′′′′:4′′′,7′′′′′′′:4′′′′,7′′′′′′′′-tetrakis[7H]pyrano[2,3-c]acridine] (the CAS index name; note that multiple primes are not divided into groups of three) [delete spaces from name]
Page 195, P-24.7.4.1, example on this page. [modified 27 May 2020]
Delete the correction to this name
Page 196, P-24.8.1.2, example.
For 2λ6-thia-4λ4-thiaspiro[5.5]undecane
read 2λ6,4λ4-dithiaspiro[5.5]undecane
Page 196, P-24.8.1.3.1, line 3.
For ...an acyclic ring system...
read ...an alicyclic system...
Page 198, P-24.8.2, example 1.
For 2λ4-2,2′-spirobi[[1,3,2]benzodioxathiole] (PIN)
read 2λ4,2′-spirobi[[1,3,2]benzodioxathiole] (PIN)
Page 199, P-24.8.2, last example.
For 1H-2λ5-2,2′-spirobi[[1,3,2]-benzodiazaphosphinine] (PIN)
read 1H-2λ5,2′-spirobi[[1,3,2]benzodiazaphosphinine] (PIN)
Page 199, P-24.8.3, example.
For 2λ6-2,2′,2′′-spiroter[[1,3,2]benzodioxathiole] (PIN)
read 2λ6,2′,2′′-spiroter[[1,3,2]benzodioxathiole] (PIN)
Page 200, P-24.8.4.1, example 3.
For 3H-2λ5-spiro[[1,3,2]benzoxazaphosphole-2,2′-[1,3,2,5]diazadiphosphinine] (PIN)
read 3H-2λ5,5′λ5-spiro[[1,3,2]benzoxazaphosphole-2,2′-[1,3,2,5]diazadiphosphinine] (PIN)
Page 202, P-24.8.5, example.
For 1′H,3′H -1λ4,1′′λ4-dispiro[thiane-1,2′-benzo[1,2-c:4,5-c′]dithiophene-6′,1′′-thiolane] (PIN)
read 1′H,3′H-1λ4,1′′λ4-dispiro[thiane-1,2′-benzo[1,2-c:4,5-c′]dithiophene-6′,1′′-thiolane] (PIN) [remove space from name]
Page 202, P-24.8.6, example 1. [modified 8 May 2019]
For 1′′λ6-dispiro[bis([1,3,2]-benzodioxathiole)-2,1′′:2′,1′′-thiopyran-4′′,1′′′-cyclopentane] (PIN)
read 1′′λ6-dispiro[bis([1,3,2]benzodioxathiole)-2,1′′:2′,1′′-thiopyran-4′′,1′′′-cyclopentane] (PIN)
replace structure with:
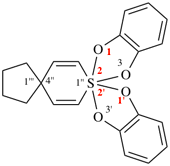
Page 206, P-25.1.1, table 2.7.
Add entries (11) and (12)
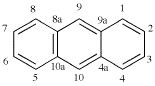 | 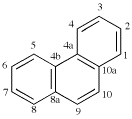 |
| (11) anthracene (PIN) (special numbering) | (12) phenanthrene (PIN) (special numbering) |
Page 211, P-25.2.1, Table 2.8 entry (3).
For (1H-isomer shown)
read (1H-isomer shown; the PIN is 1H-perimidine)
replace structure with:
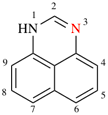
Page 211, P-25.2.1, Table 2.8 entry (4).
For (4) acridine* (PIN)
read (4) acridine* (PIN)
(special numbering)
Page 212/3, P-25.2.1, Table 2.8 entries (13), (14) and (15). [modified 11 November 2020]
For *
read *†
Page 214, Table 2.8, Structure (23), line 16.
For tellurochromene (2H-isomer shown)
read tellurochromene (Te instead of O) (2H-isomer shown)
Page 214, P-25.2.1, Table 2.8 footnote.
For * In CAS index nomenclature, quinolizine precedes quinoline in seniority.
read † In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
Page 214, P-25.2.1, Table 2.9.
For isoquinoline (PIN), isoarsinoline (PIN), and isophosphinoline (PIN)
read isoquinoline† (PIN), isoarsinoline (PIN), and isophosphinoline (PIN)
for quinoline (PIN), arsinoline (PIN), and phosphinoline (PIN)
read quinoline† (PIN), arsinoline (PIN), and phosphinoline (PIN)
for quinolizine (PIN), arsinolizine (PIN), and phosphinolizine (PIN)
read quinolizine† (PIN), arsinolizine (PIN), and phosphinolizine (PIN)
add footnote † In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
Page 216, P-25.2.2.2, example 1 in the left column. [corrected 30 January 2019]
Delete dibenzo[1,4]dioxine)
Page 217, P-25.2.2.3, first example on page.
For phenoazaphosphinine
read phenazaphosphinine
Page 217, P-25.2.2.4, lines 1/2. [corrected 22 April 2020]
For Unless listed as a retained name in Table 2.8, for example quinoline and cinnoline, a benzene ring fused to a heteromonocycle of five or more members (a benzoheterocycle) is named...
read If the initial identified preferred components for naming a fused ring system include an isolated benzo component (i.e. not forming part of a component with a retained name such as quinoline or anthracene) ortho-fused to a heteromonocyclic component of five or more members, these two components are treated together as a one-component unit (a benzoheterocycle). See P-25.3.5 for the use of benzoheterocycles in fusion nomenclature and limitations on its use.. It is named....
Page 218, P-25.2.2.4, example 1. [corrected 26 January 2022]
Delete 3-benzooxepine
Page 218, P-25.2.2.4, example 2. [corrected 26 January 2022]
Delete 4H-3,1-benzooxazine
Page 218, P-25.2.2.4, last example.
For benzo[j][1,5,8]cyclooxathiaazadodecine
read benzo[j][1,8,5]oxathiaazacyclododecine
Page 221, P-25.3.1.3, example 1.
Replace structure for azulene with:
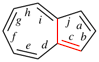
Page 222, P-25.3.1.3, example 1 on this page.
Replace structure for azulene with:

Page 222, P-25.3.1.3, left example at bottom of page.
Replace the structure with:
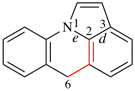
Page 226, P-25.3.2.3.1, line 6. (corrected 1 December 2021)
For ...horizontal rows. Such rows...
read ...horizontal rows. If the compound requires distorted rings not shown below see P-25.3.2.3.2. Such rows...
Page 226, P-25.3.2.3.1, before the example add. [corrected 23 February 2022)
Add 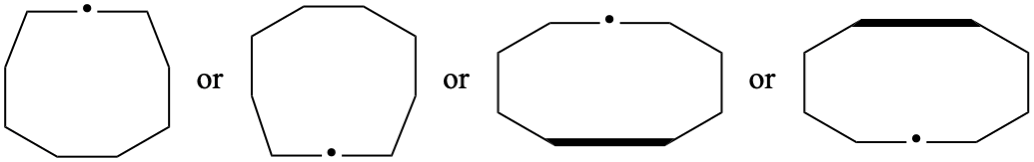
Page 226, P-25.3.2.3.2. (corrected 1 December 2021)
For P-25.3.2.3.2
read P-25.3.2.3.3
Page 226, insert new P-25.3.2.3.2 after P-25.3.2.3.1 (corrected 1 December 2021)
P-25.3.2.3.2 Distorted ring shapes
If a compound cannot be drawn only using the shapes shown in P-25.3.2.3.1 a distorted ring shape will be required. The distorted ring should be as small as possible.
 | not | 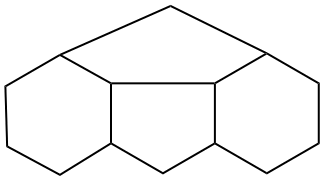 |
| only preferred shapes used | distorted five-membered ring | |
 | not | 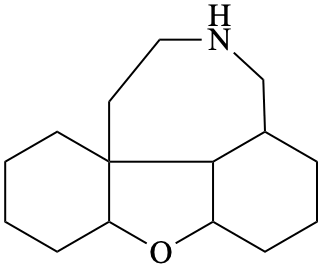 |
| only preferred shapes used (the separation to show the seven-membered ring is not fused to the left hand ring does not count as a distortion) | distorted seven-membered ring | |
 | not |  |
| distorted four-membered ring | distorted six-membered ring |
Page 229, P-25.3.2.3.2 (d), structure 3.
For ¾ ring in lower left
read ¾ ring in lower left quadrant
Page 231, P-25.3.2.4 (c), example 1. [corrected 24 October 2018]
Replace the structure with:
Page 231, P-25.3.2.4 (e), example.
Replace the structure with:
Page 232, P-25.3.2.4 (f), example 1. [corrected 24 October 2018]
Replace the structure with:
Page 233, P-25.3.2.4 (j), example. (modified 23 October 2019)
Replace the diagram with:
for indeno[1,7-kl]aceanthrylene
read acephenanthryleno[5,4-k]aceanthrylene
Page 234, P-25.3.2.5.2, line 2.
For ...convention (see P-14.1) The...
read ...convention (see P-14.1.3). The...
Page 234, P-25.3.2.5.2, example.
Replace the structure with:
Page 235, P-25.3.3.1.1, example 1.
Replace the structure with:
Page 237, P-25.3.3.1.2 (c) example 2. (corrected 23 October 2019)
Replace the structures with:
Page 237, P-25.3.3.1.2 (d), example.. [corrected 12 May 2021]
For [1,3]diazeto[1,2-a:3,4-a′]dibenzimidazole (PIN)
read [1,3]diazeto[1,2-a:3,4-a′]dibenzimidazole
add [1,3]diazeto[1,2-a:3,4-a′]bis([1,3]benzimidazole) (PIN)
Page 238, P-25.3.3.1.2 (f), example 2, right version. [corrected 10 October 2018]
Replace the structure with:
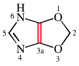
Page 239, P-25.3.3.2.2, example.
For pyrazino[2,1,6-cd:3,4,5-c′,d′]dipyrrolizine
read pyrazino[2,1,6-cd:3,4,5-c′d′]dipyrrolizine
Page 239, P-25.3.3.2.3, example
Replace the structure with:
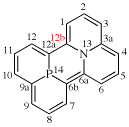
Page 240, P-25.3.3.3.1, example 3.
For 2H,6H-quinolizino[3,4,5,6,7-defg]acridine (PIN)
read 2H,6H-quinolizino[3,4,5,6,7-defg]acridine (PIN)
(recommended numbering)
Page 242, P-25.3.4.1.2, example 1.
Replace the structure with:
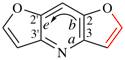
Page 246, P-25.3.4.2.2 (a) example. [corrected 19 September 2018]
For 8H-cyclopenta[3,4]napththo[1,2-d][1,3]oxazole (PIN)
read 8H-cyclopenta[3,4]naphtho[1,2-d][1,3]oxazole (PIN)
replace the structure with:
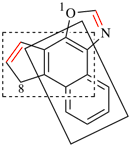
Page 248, P-25.3.4.2.3.1, example 3.
Delete Example:
replace the structure with:
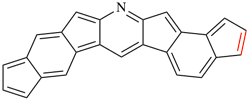
Page 253, P-25.3.4.2.4(h) example.
Replace structure with:
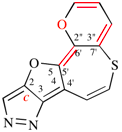
Page 254, P-25.3.5.4, example 2.
For (not [1]benzofuro[5′,4′:3,4]cyclopenta[1,2-b]pyridine; pridine is senior to furan; indene...
read (not [1]benzofuro[5′,4′:3,4]cyclopenta[1,2-b]pyridine; pyridine is senior to furan; indene...
Replace the structure with:
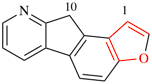
Page 255, P-25.3.5.4, example 1 on this page. [corrected 30 January 2019]
For 6H-benzo[b][2]benzopyran
read 6H-benzo[c][2]benzopyran
Page 255, P-25.3.5.4, example on this page.
For dibenzo[b,d]pyran (PIN)
read 6H-dibenzo[b,d]pyran (PIN)
Page 255, P-25.3.6.1, example 3.
For dibenzo[g,p][1,3,6,9,12,15,18]heptaoxacycloicosine (PIN)
read 9H-dibenzo[g,p][1,3,6,9,12,15,18]heptaoxacycloicosine (PIN)
Page 256, P-25.3.6.1, last example.
Replace the structure with:
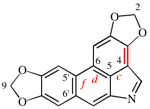
Page 258, P-25.3.7.1, example 5.
For cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiazine)
read cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiine)
Page 262, P-25.3.8.3, example. [corrected 14 November 2018]
For furo[3′,4′;5,6]pyrazino[2,3-c]pyridazine (PIN)
read furo[3′,4′:5,6]pyrazino[2,3-c]pyridazine (PIN)
replace the structure with:
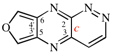
Page 262, P-25.3.8.4, example 3. [corrected 3 October 2018]
For naphtho[1,8a]azirine (PIN)
read naphtho[1,8a-b]azirine (PIN)
replace the structure with:
Page 265, P-25.4.1.9, example.
Replace the structure with:
Page 266, P-25.4.2.1.2, replace the structure of example 1. [corrected 26 January 2022]
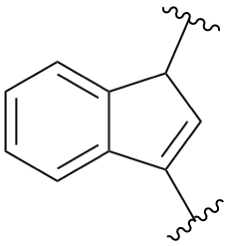
Page 266, P-25.4.2.1.2, replace structure of example 2. [corrected 26 January 2022]
Page 267, P-25.4.2.1.3, replace structure of example 2 on this page. [corrected 26 January 2022]
Page 267, P-25.4.2.1.4, line 3 of box.
For ...‘azano’, –NH–; –N=N–; epitriazano, –NH-NH-NH–; and –NH-N=N–, epitriaz[1]eno. ...
read ...‘azano’, –NH–; ‘diazeno’, –N=N–; ‘epitriazano’, –NH-NH-NH–; and –NH-N=N–, ‘epitriaz[1]eno’. ...
Page 271, P-25.4.2.3.2, example 4.
For epoxyethane[1,1,2]triyl (preferred prefix)
read (epoxyethane[1,1,2]triyl) (preferred prefix)
Replace the diagram with:
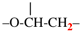
Page 272, P-25.4.2.3.2, example 1 on this page.
For epoxyethane[1,2,2]triyl (preferred prefix)
read (epoxyethane[1,2,2]triyl) (preferred prefix)
Replace the diagram with:
–O-CH2-CH<
Page 273, P-25.4.3.2.1, Example 2. [corrected 30 January 2019]
For 1,4:6,9-dimethanodibenzo[b,e][1,4]dioxin
read 1,4:6,9-dimethanodibenzo[b,e][1,4]dioxine
Page 273, P-25.4.3.2.1, example 3. [modified 27 May 2020]
For 6,9-epoxy-1,4-methanobenzocyclooctene (PIN)
read 6,9-epoxy-1,4-methanobenzo[8]annulene (PIN)
Page 274, P-25.4.3.2.2, example 1. [modified 11 December 2019]
For 7H-3,5-(epoxymethano)furo[2,3-c]pyran (PIN)
read 2H-3,5-(epoxymethano)furo[3,4-b]pyran (PIN) (I)
add (not 7H-3,5-(epoxymethano)furo[2,3-c]pyran) (II) [preferred ring system, see P-25.4.3.4.2 (d)]
replace the structure with:
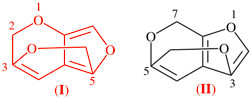
Page 274, P-25.4.3.2.2, example 2. [modified 11 December 2019]
For 7H-5,3-(epoxymethano)furo[2,3-c]pyran (PIN)
read 7H-5,3-(epoxymethano)furo[2,3-c]pyran (PIN) (I)
add (not 4H-6,1-(epoxymethano)furo[3,4-c]pyran) (II)
[preferred ring system, see P-25.4.3.4.2 (d)]
replace the structure with:
Page 277, P-25.4.3.4.2, (c)
Replace the example with:
 | not |  |
| 1,4:8,5-bis(epoxymethano)anthracene (I) (PIN) | [not 1H,3H-1,4:6,9-bisethenobenzo[1,2-c:5,4-c′]dipyran (II)] |
Page 279, P-25.4.3.4.2 (h), example. [modified 27 May 2020]
For 8,7-(azenoetheno)cyclohepta[4.5]cycloocta[1,2-b]pyridine (I) (PIN)
read 8,7-(azenoepietheno)cyclohepta[4.5]cycloocta[1,2-b]pyridine (I) (PIN)
for [8,7-(azenoepiethanediylidene)cyclohepta[4,5]cycloocta[1,2-b]pyridine (II);
read [not 8,7-(azenoepiethanediylidene)cyclohepta[4,5]cycloocta[1,2-b]pyridine (II);
Page 280, P-25.4.3.4.2 (i), example. (modified 23 October 2019)
Replace the diagram with:

for 5,14-(metheno)-2,3,4-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene (PIN);
read 14,5-(metheno)-3,2,4-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene (PIN)
for the single bond of the metheno group is given preference the lower locant of the ring system (see P-25.4.3.2.2)]
read the locants set for the independent bridge ‘3,2,4’ is the lowest; the locant for single bond attachment of metheno bridge is cited first.
for [not 5,14-(metheno)-2,4,3-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene
read [not 5,14-(metheno)-3,4,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
for (the locants ‘2,3,4’ for the independent bridge are lower than ‘2,4,3’)]
read not 14,5-(metheno)-4,2,3-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
for [not 14,5-(metheno)-2,3,4-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene
read not 5,14-(metheno)-4,3,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
for (the locants '5,14' for the independent bridge are lower than '14,5')]
read (the locants ‘3,2,4’ for independent bridge is lower than ‘3,4,2’, ‘4,2,3’ and ‘4,3,2’]
Page 282, P-25.4.4.1, example 2 on this page.
Replace the structure with:
Page 283, P-25.4.5.1, example. [corrected 10 October 2018]
Replace the structure with:
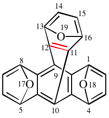
Page 283, P-25.4.5.2, example 1. (corrected 23 October 2019)
Replace the structures with:
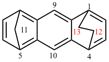
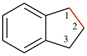
Page 285, P-25.5.1.1, example 1. [corrected 14 November 2018]
For 1,2,3,4,5,6-hexaazacyclopenta[c,d]pentalene (PIN)
read 1,2,3,4,5,6-hexaazacyclopenta[cd]pentalene (PIN)
Page 287, P-25.5.3, example 1.
Replace the left structure with:
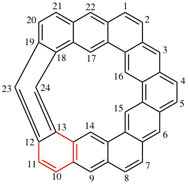
Page 288, P-25.6, example 1. [corrected 27 May 2020]
For 7λ4-[1,2]dithiolo[1,5-b][1,2]dithiole (PIN)
read 7λ4-[1,2]dithiolo[5,1-e][1,2]dithiole (PIN)
Page 290, P-25.7.1.1, example 3 on this page.
For 2H-2λ5-2,6-(ethanylylidene)isophosphinoline (PIN)
read 2H-2λ5-2,6-(epiethanylylidene)isophosphinoline (PIN)
Page 294, P-25.8.1, structure. (corrected 23 October 2019)
Add 10H-phenoxazine
Page 294, P-25.8.1, Note 3. [modified 11 August 2021]
For In the order of seniority used by CAS quinolizine precedes quinoline and the indacenes precede biphenylene.
read In the order of seniority used by CAS quinolizine precedes quinoline and isoquinoline; and indolizine preceeds indole and isoindole.
Page 296, P-25.8.1, line 12. [corrected 30 January 2019]
For selanthrene
read selenanthrene
Page 297, P-25.8.2, after line 6. [corrected 11 December 2019]
Add (5) In the order of seniority used by CAS the indacenes precede biphenylene.
Page 298, P-25.8.2, line 7 on this page.
For fluoranthrene C6C6C5C5
read fluoranthrene C6C6C6C5
Page 298, P-26.0, line 3. [corrected 8 May 2019]
For ...recommendations contained therein.
read ...recommendations contained therein except a change in the usage of brackets for heterocyclic amplificants which require locants. When a phane system contains a heterocyclic amplificant with locants, they are enclosed in brackets (see P-26.4.2.2, example 1 and P-26.5.1, example 2).
Page 299, P-26.1.1, line 1.
For ...illustrated in Fig. 1. The...
read ...illustrated in Fig. 2.1. The...
Page 299, P-26.1.1, lines 10/11.
For ...in Fig. 1 do not...
read ...in Fig. 2.1 do not...
Page 302, P-26.2.2.1, example 2.
For pyran (PIN) furana (preferred prefix)
read pyran (PIN) pyrana (preferred prefix)
add furan (PIN) furana (preferred prefix)
Page 302, P-26.2.2.2.2 (a) (2). [corrected 8 May 2019]
For spiro[1,3-dioxolane-2,1′-indene]
read spiro[[1,3]dioxolane-2,1′-indene]
Page 303, P-26.2.4.2, heading.
For P-26.2.4.2
read P-26.2.3.2
Page 304, P-26.3.2.1, Example.
For 1,4(1,4)-dibenzenacycloheptaphane (PIN)
read 1,4(1,4)-dibenzenacyclohexaphane (PIN)
for [not 1(1,4),4(1,4)-dibenzenacycloheptaphane
read [not 1(1,4),4(1,4)-dibenzenacyclohexaphane [change to justify the cross-reference]
Page 304, P-26.3.2.2, example. [corrected 8 May 2019]
For [not 1(1,3)-benzena-4(1,4)-benzenacycloheptaphane; see second example of P-26.4.2.2]
read [not 1(1,4),4(1,3)-dibenzenacycloheptaphane; see second example of P-26.4.2.2]
Page 305, P-26.4.1.2, last example.
Replace simplified skeleton structure with:
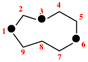
for ...locant set ‘1,5,7’ in either of the other names. see P-26.4.2.1]
read ...locant set ‘1,3,7’ or ‘1,5,7’ in either of the other names, see P-26.4.1.1]
Page 307, P-26.4.1.4, example 2.
Replace the structure with:
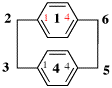
Page 308, P-26.4.1.4, example 2 on this page.
For 3(2,5)-furana-1,7(1)5(1,4)-tribenzenaheptaphane (PIN)
read 3(2,5)-furana-1,7(1),5(1,4)-tribenzenaheptaphane (PIN)
for [not 3(5,2)-furana-1,7(1),5(1,4)-tribenzenaheptaphane;
read [not 3(5,2)-furana-1,7(1),5(1,4)-tribenzenaheptaphane;
for for superatom 3 the attachment locant set ‘2,5’ for the PIN is lower than ‘5,2’]
read for superatom 3 the lower attachment locant is not adjacent to the lower-numbered atom 2 of the simplified skeleton]
Page 309, P-26.4.2.2, example 1. [corrected 27 May 2020]
For {not 1,9(2,4)-diquinolina-3(5,3),7(2,4)-dipyridina-5(3,5)- [1,2]oxazolacyclotetradecaphane;
read {not 1(2,4),9(4,2)-diquinolina-3(5,3),7(2,4)-dipyridina-5(3,5)- [1,2]oxazolacyclotetradecaphane;
Page 310, P-26.4.2.2, example 2 on this page.
Replace the structure with:
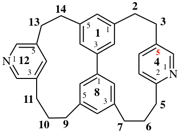
Page 311, P-26.4.2.3, example 1.
Replace the diagram with:
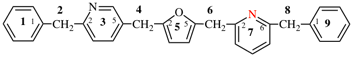
Page 313, P-26.4.2.4, example 2. [corrected 19 September 2018]
For [not 3(10,3)-phenanthrena-6(5,8,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecane
read [not 3(10,3)-phenanthrena-6(5,8,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecaphane
Page 315, P-26.4.3.3, example 2 on this page. [corrected 13 February 2019]
For [not 14,16,45,46-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane; the...
read [not 14,16,44,45-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane; the...
for ...‘14,15,44,46 ’, is lower than ‘14,16,45,46 ’ (see P-26.4.3.3)]
read ...‘14,15,44,46 ’, is lower than ‘14,16,44,45 ’]
Page 315, P-26.4.3.4, example.
For 14H-1(3,5)pyrana-4(1,3)-benzenacyclohexaphane (PIN)
read 14H-1(3,5)-pyrana-4(1,3)-benzenacyclohexaphane (PIN)
Page 320, P-26.5.4.1, example 1 on this page.
For 1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
read
6-oxa-2-thia-4-selena-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
for [not 7(4)-pyridina-1(1),3,5(1,4)-tribenzenaheptaphane;
read [not 2-oxa-6-thia-4-selena-7(4)-pyridina-1(1),3,5(1,4)-tribenzenaheptaphane;
Page 321, P-26.5.4.2, example on this page. [corrected 30 March 2022]
For Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)dicyclotetradecanacyclododecaphane (I) (PIN)
read Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (I) (PIN)
Page 321, P-26.5.4.2, example on this page.
For [not 114,2,4,54,6,12-hexaaoxa-14,514-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (II);...
read [not 114,2,4,54,6,12-hexaoxa-14,514-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (II);...
Page 322, P-27.0, lines 1/2.
For This section is based on the publication ‘Phane nomencature, Part 1 Phane parent names’ (ref. 5) and the new edition 2012 contains...
read This section is based on the publication ‘Nomenclature for the C60-Ih and C70-D5h(6) Fullerenes’ (ref. 10) and the new edition 2013 contains...
Page 324, P-27.2.1, lines 4/5. [corrected 9 January 2019]
For ...name (C70-I5h(5))[5,6]fullerene (PIN).
read ...name (C70-I5h(5))[5,6]fullerene (PIN).
Page 324, P-27.2.2, lines 1/2. [corrected 18 September 2019]
For ...and [70-D5h(6)]fullerene in usage)...
read ...and [70]fullerene in usage)...
Page 324, P-27.3, line 4. [corrected 3 October 2018]
For ...(PIN) and (C70-D5h(6))-[5,6]fullerene (PIN)...
read ...(PIN) and (C70-D5h(6))[5,6]fullerene (PIN)...
Page 326, P-27.3 example 1 on this page. [corrected 19 September 2018]
For [60]fullerene (trivial numbering)
read [C60-Ih]fullerene (trivial numbering)]
Page 326, P-27.3 example 2 on this page. [corrected 3 October 2018]
For [C70-D5h]fullerene (trivial numbering)
read [C70-D5h]fullerene (trivial numbering)]
Page 335, P-27.6.1, example 1 on this page. [modified 8 May 2019]
For 3′H,3′′H,3′′′H-tricyclopropa[7,22:33,34:46,47](C70-Ih)[5,6]fullerene (PIN)
read 3′H,3′′H,3′′′H-tricyclopropa[7,22:33,34:46,47](C70-D5h(6))[5,6]fullerene (PIN)
for 7,22:33,34:46,47-tris(methylene)(C70-Ih)[5,6]fullerene (see P-27.6.2)
read 7,22:33,34:46,47-tris(methylene)(C70-D5h(6))[5,6]fullerene (see P-27.6.2)
Page 337, P-27.6.2 example 1 on this page. [corrected 9 January 2019]
For ...decanor(C60- Ih)[5,6]fullerene (PIN)
read ...decanor(C60-Ih)[5,6]fullerene (PIN) [delete space from name]
Page 337, P-27.6.2, example 2 on this page (example 4 of section). [modified 9 April 2025]
For 1,9:32,33-(ethane-1,2,2-triyloxymethylene-1,4-phenylenemethyleneoxyethane-1,2,2-triy)(C60-Ih)[5,6]fullerene
read 1,9:32,33-[ethane-1,1,2-triyloxymethylene(1,4-phenylene)methyleneoxyethane-1,2,2-triyl](C60-Ih)[5,6]fullerene
Page 338, P-27.6.3, example. [modified 24 October 2018]
For spiro[cyclohexane-1,1′a-[1(9a)H-1(9)a]-homo(C60-Ih)[5,6]fullerene] (PIN)
read spiro[cyclohexane-1,1′a-[1(9)a]homo(C60-Ih)[5,6]fullerene] (PIN)
replace the structure with:
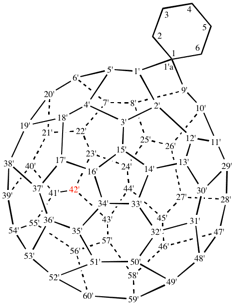
Page 340, P-28.2.1, example 4.
Replace the structure with:
Page 341, P-28.2.2, example 2. [corrected 31 July 2019]
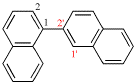
For 2,2′-bi(bicyclo[2.2.1]heptan-2-ylidene) (PIN)
read 2,2′-bi(bicyclo[2.2.1]heptanylidene) (PIN)
Page 341, P-28.2.3, example 1.
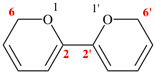
For 2H,2′H-6,6′-bipyran (PIN)
read 6H,6′H-2,2′-bipyran (PIN)
for
(not 6,6′-bi-2H-pyran)
read (not 2,2′-bi-6H-pyran)
replace the structure with:
Page 341, P-28.2.3, paragraph 3, lines 6-7. (corrected 1 December 2021)
For ...requiring a bivalent substituent group...
read ...requiring a divalent substituent group...
Page 342, P-28.2.3, example 4 from top.
For 1,2′-biindole
read 2′H-1,2′-biindole
delete the phrase (no indicated hydrogen needed)
Page 342, P-28.2.3, example 5 on this page.
Delete (not 4,4′-bi-1H-azepine)
Page 343, P-28.3.1, Note, lines 5/6.
For ... two alternative names are 1,1′,2′,1′′-tercyclopropane and 11,21,22,31-tercyclopropane, where the ...
read ... two alternative names are 1,1′:2′,1′′-tercyclopropane and 11,21:22,31-tercyclopropane, where the ...
Page 344, P-28.3.1, example 1 on this page. [corrected 23 January 2019]
For (not 1,1′:3′,1′′:2′,1′′′-quatercyclobutane; ...
read (not 1,1′:3′,1′′:2′′,1′′′-quatercyclobutane; ...
Page 344, P-28.3.1, example 3 on this page.
For 11H-13,23:23,33-terindole
read 11H,33H-13,23:23,33-terindole
for 1H-3,3′:3′,3′′-terindole
read 1H,3′′H-3,3′:3′,3′′-terindole
Page 344, P-28.3.1, last example.
For 2H,2′H,4′H,2′′H-1,1′3′,1′′-terpyrimidine
read 2H,2′H,2′′H,4′H-1,1′:3′,1′′-terpyrimidine
Page 345, P-28.4.1, example 2.
For 3′,3′′,13′-trioxa-1,1′:7′,1′′-tercyclotetradecane
read 3,3′′,13′-trioxa-1,1′:7′,1′′-tercyclotetradecane
replace the diagram with:

Page 346, P-28.4.1, example 2 on this page. [corrected 19 September 2018]
For 2,2′-dithia-1,1′-bi(cyclododecan-2-ylidene) (PIN)
read 2,2′-dithia-1,1′-bi(cyclododecylidene) (PIN)
Page 347, P-28.5, example 2. [modified 30 January 2019]
For 1,8(2),2,3,4,5,6,7(2,5)-heptathiophenaoctaphane (PIN)
read 1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
for 12,22:25,32:35,42:45,52:55,62:65,72,75,82-octithiophene
read 12,22:25,32:35,42:45,52:55,62:65,72:75,82-octithiophene
Page 347, P-28.5, example 3. [modified 30 March 2022]
For 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)heptapyrrolaheptaphane (PIN)
read 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)-heptapyrrolaheptaphane (PIN)
for 1H,1′H,1′′H,1′′′H,1′′′ ′H,1′′′ ′′,1′′′ ′′′H-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′-septipyrrole
read 1H,1′H,1′′H,1′′′H,1′′′ ′H,1′′′ ′′H,1′′′ ′′′H-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′-septipyrrole
Page 348, P-28.6, example 2.
For 3′′-([1,1′-biphenyl]-3-yl)-1,1′:3′1′′:3′′-1′′′:3′′′,1′′′′-quinquephenyl
read 5′′-([1,1′-biphenyl]-3-yl)-1,1′:3′,1′′:3′′,1′′′:3′′′,1′′′ ′-quinquephenyl
Page 351, P-29.1.2, example 2. [corrected 11 November 2020]
For chlorocarbonyl (preferred prefix)
read carbonochloridoyl (preferred prefix)
Page 356, P-29.3.2.2, example 7 on this page.
Replace the diagram with:
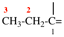
Page 356, P-29.3.2.2, example 10 on this page.
For AsH3-AsH2–
read AsH2-AsH–
Page 357, P-29.3.2.2, example 1 on this page.
For disilylamino (preferred prefix)
read disilylamino (preselected prefix)
Page 358, P-29.3.4.1, line 4.
For ‘added inducated hydrogen’
read ‘added indicated hydrogen’
Page 361, P-29.3.5, example 3 on the page.
For 1,1′:3′,1′′]-terphenyl]-4,4′-diyl
read [1,1′:3′,1′′-terphenyl]-4,4′-diyl
Page 362, P-29.3.6, example (I)
Replace the structure with:
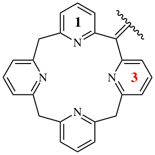
Page 363, P-29.4.1, example 4 on this page.
Replace the structure with:
Page 364, P-29.4.2, example 1.
For silanediyldiethane-2,1-diyl (preferred prefix)
read silanediyldi(ethane-2,1-diyl) (preferred prefix)
Page 364, P-29.4.2, example 5. [corrected 23 January 2019]
For silanediylbis(1-fluoroethylene)
read [not silanediylbis(1-fluoroethylene)]
Page 366, P-29.6.1, example 3. [modified 18 September 2019]
For tert-butyl(dimethyl)phosphane (PIN)
read tert-butyldi(methyl)phosphane (PIN)
for dimethyl(1-methylethyl)phosphane
read (1,1-dimethylethyl)di(methyl)phosphane
Page 366, P-29.6.1, example 3. [modified 20 February 2019]
for dimethyl(1-methylethyl)phosphane
read (1,1-dimethylethyl)dimethylphosphane
Page 366, P-29.6.1, example 4.
For (2-chloro-2,2-dimethylethyl)silane
read (2-chloro-1,1-dimethylethyl)silane
Page 367, P-29.6.2.1, example 4.
For 3,4,5-trimethylbenzylidyne (preferred prefix)
read 3,4,5-trimethylbenzylidyne
for (3,4,5-trimethylphenyl)methylidyne
read (3,4,5-trimethylphenyl)methylidyne (preferred prefix)
Page 368, P-29.6.2.2, example 1.
Replace the structure with:
Page 368, P-29.6.2.2, example 4.
For trityl (no substitution allowed) (preferred prefix)
read trityl (no substitution allowed)
for triphenylmethyl
read triphenylmethyl (preferred prefix)
Page 368, P-29.6.2.2, example 5. [corrected 11 November 2020]
For (4-methylphenyl)diphenylmethyl (preferred prefix)
read (4-methylphenyl)di(phenyl)methyl (preferred prefix)
Page 369, P-29.6.2.3, example 2 on this page.
For anthacen-2-yl (preferred prefix)
read anthracen-2-yl (preferred prefix)
Page 369, P-29.6.2.3, example 4 on this page.
For 7-isoquinolyl (also 1-, 3-, 5-, 6-, 7-, and 8-isomers)
read 7-isoquinolyl (also 1-, 3-, 4-, 5-, 6- and 8-isomers)
for isoquinolin-7-yl (also 1-, 3-, 5-, 6-, 7-, and 8-isomers; preferred prefixes)
read isoquinolin-7-yl (also 1-, 3-, 4-, 5-, 6- and 8-isomers; preferred prefixes)
Page 369, P-29.6.2.3, example 5 on this page.
For 2-naphthyl (also 1- isomer
read 2-naphthyl (also 1-isomer)
Page 369, P-29.6.2.3, example 8 on this page.
For pyridin-2-yl (also 3- and 4-, preferred prefixes)
read pyridin-2-yl (also 3- and 4-isomers; preferred prefixes)
Page 370, P-29.6.2.3, example 2 on this page.
For 2-thienyl (also 3-isomer) (also 3-isomer; preferred prefixes)
read 2-thienyl (also 3-isomer)
for thiophen-2-yl (also 3-isomer)
read thiophen-2-yl (also 3-isomer; preferred prefixes)
Page 371, P-29-6.3, example 3 on this page.
For furan-2-ylmethyl (preferred prefix)
read (furan-2-yl)methyl (preferred prefix)
Page 371, P-29-6.3, example 4 on this page.
For thiophen-2-ylmethyl (preferred prefix)
read (thiophen-2-yl)methyl (preferred prefix)
Page 374, P-31.1.1.1, after text. [corrected 4 September 2024]
Add If the stereochemistry of a double bond is indicated in a structure it should be specified by E or Z with the appropriate locant. This includes double bonds in rings of eight or more members (see P-91.2.2).
Page 374, P-31.1.1.1, example 2. [corrected 19 September 2018]
For prop-1-yne (PIN)
read propyne (PIN)
Page 374, P-31.1.1.1, last example.
Replace the diagram with:
Page 375, P-31.1.2.2.1, structure 1.
Replace structure with:
Page 375, P-31.1.2.2.1, structure 3.
Replace structure with:
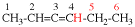
Page 377, P-31.1.3.1, example 2 on this page. [corrected 4 September 2024]
For cyclododeca-1,5-diene (PIN)
read (1Z,5E)-cyclododeca-1,5-diene (PIN)
Page 377, P-31.1.3.1, example 3 on this page. [corrected 4 September 2024]
Replace the structure with:

for cyclopentadec-1-en-4-yne (PIN)
read (1Z)-cyclopentadec-1-en-4-yne (PIN)
Page 377, P-31.1.3.2, example 1. [corrected 4 September 2024]
For1,4,7,10-tetraoxacyclododec-2-ene (PIN)
read (2Z)-1,4,7,10-tetraoxacyclododec-2-ene (PIN)
Page 377, P-31.1.3.2, example 2. [corrected 4 September 2024]
For 1-oxa-4-azacyclododec-3-ene (PIN)
read (3E)-1-oxa-4-azacyclododec-3-ene (PIN)
Page 377, P-31.1.3.2, example 3. [corrected 4 September 2024]
For 1,11-disilacycloicosa-5,7-dien-3-yne (PIN)
read (5Z,7E)-1,11-disilacycloicosa-5,7-dien-3-yne (PIN)
Page 378, P-31.1.3.2, example 1 on this page. [corrected 4 September 2024]
For 1,10-disilacycloicosa-12,14,16-trien-18-yne (PIN)
read (12Z,14E,16Z)-1,10-disilacycloicosa-12,14,16-trien-18-yne (PIN)
Page 378, P-31.1.3.3, examples.
Delete n = 11:
Replace the structures with:
Page 379, P-31.1.3.4, example 2.
For 1,1′-ethene-1,2-diyldibenzene (PIN)
read 1,1′-(ethene-1,2-diyl)dibenzene (PIN)
Page 380, P-31.1.4.2, example 2.
For [not bicyclo[6.5.1]tetradec-1(13)-decene]
read [not bicyclo[6.5.1]tetradec-1(13)-ene]
Page 381, P-31.1.4.2 (2), example 2. [modified 30 October 2019]
For tricyclo[9.3.1.14,8]hexadeca-1(15),4(16),5,7,11,13-hexaene (I) (PIN)
read tricyclo[9.3.1.14,8]hexadeca-1(15),4(16),5,7,11,13-hexaene (I)
for tricyclo[9.3.1.14,8]hexadeca-1(14),4(16)5,7,11(15),12-hexaene (IV
read tricyclo[9.3.1.14,8]hexadeca-1(14),4(16),5,7,11(15),12-hexaene (IV);
Page 382/383, P-31.1.4.3. [corrected 4 September 2024]
The order of the three criteria changed to make minimum number of compound locants first.
Page 382, P-31.1.4.3 (2) formerly (1), example. [corrected 4 September 2024]
For bicyclo[14.3.1]icosa-11,13,18-trien-2-yne (PIN)
read (11E,13E)-bicyclo[14.3.1]icosa-11,13,18-trien-2-yne (PIN)
Page 382, P-31.1.4.3 (3) formerly (2), example. [corrected 4 September 2024]
For bicyclo[11.3.1]heptadeca-2-en-11-yne (PIN)
read (2Z)-bicyclo[11.3.1]heptadeca-2-en-11-yne (PIN)
Page 383, P-31.1.4.3 (1) formerly (3), example. [corrected 4 September 2024]
For bicyclo[8.3.1]tetradeca-4,6,10-trien-2-yne (PIN)
read (4Z,6E)-bicyclo[8.3.1]tetradeca-4,6,10-trien-2-yne (PIN)
Page 384, P-31.1.5.1.2 (1), example. [corrected 4 September 2024]
For spiro[4.10]pentadec-10-en-8-yne (PIN)
read (10Z)-spiro[4.10]pentadec-10-en-8-yne (PIN)
Page 384, P-31.1.5.1.2 (2), example. [corrected 4 September 2024]
For spiro[4.10]pentadec-6-en-14-yne (PIN)
read (6E)-spiro[4.10]pentadec-6-en-14-yne (PIN)
Page 386, P-31.1.6.1, (1), (b) and (2). [changed 20 November 2024]
For (b) composite locants, neither of which is adjacent to a primary locant;
read (b) composite locants;
for (2) by a compound locant, when one locant is a composite locant adjacent to a primary locant.
read (2) by a compound locant, when two locants are not consecutive, or when one is a primary locant and the other is a composite locant.
Page 386, P-31.1.6.1 before examples. [added 20 November 2024]
Add in a box This is a change from PhII-5.3.2.
Page 386, P-31.1.5.2.1, example on this page. [corrected 27 May 2020]
For 2′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]hexan]-4′′-ene (PIN)
read 2′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-4′′-ene (PIN)
for [not 5′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]hexan]-2′′-ene;
read [not 5′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-2′′-ene;
Page 386, P-31.1.6.1, before examples on next page. [corrected 4 September 2024]
Add If there is a choice the criteria of P-31.1.4.3 are applied.
Page 387, P-31.1.6.1, example 2. [corrected 27 May 2020]
For 1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93(94)-diene (PIN)
read 1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93-diene (PIN)
Page 387, P-31.1.6.1, new example after example 3. [added 20 November 2024]
Add the structure:

(2E,8(91)Z)-1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphan-2,8(91),92-triene (PIN)
Page 388 , P-31.1.6.2, example 1 on this page. [corrected 4 September 2024]
For 1,7(1,3)dibenzenacyclotridecaphan-2-en-5-yne (PIN)
read (2E)-1,7(1,3)dibenzenacyclotridecaphan-2-en-5-yne (PIN)
Page 388, P-31.1.7.1, example 1.
For [1,1′-bicyclohexane]-1,2′-diene (PIN)
read [1,1′-bi(cyclohexane)]-1,2′-diene (PIN)
Page 388, P-31.1.7.1, example 2.
For [2,2′-bibicyclo[2.2.2]octane]-5,5′-diene (PIN)
read [2,2′-bi(bicyclo[2.2.2]octane)]-5,5′-diene (PIN)
Page 390, P-31.1.7.3, example.
For 12,23,33-trithia[11,21:24,31-terbicyclo-[2.2.2]octane]-15,25,35-triene (PIN)
read 12,23,33-trithia[11,21:24,31-terbicyclo[2.2.2]octane]-15,25,35-triene (PIN)
for 2,3′,3′′-trithia-[1,1′:4′,1′′-terbicyclo-2.2.2]octane]-5,5′,5′′-triene
read 2,3′,3′′-trithia[1,1′:4′,1′′-terbicyclo[2.2.2]octane]-5,5′,5′′-triene
Page 392. P-31.2.3.2, after last example on this page.
Add:
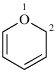 |  |
| 2H-pyran (PIN) | oxane (PIN) |
| tetrahydropyran |
Page 393, P-31.2.3.2, example 2 at top of page.
For 1,4-thiazepinane (PIN)
read 1,4-thiazepane (PIN)
Page 393, P-31.2.3.2, example 3 on this page. [modified 27 May 2020]
For pentazolidine
read pentazolidine (preselected name; see P-12.1; P-22.2.2.1.5.2, P-22.2.5)
delete pentazolane
Page 395, P-31.2.3.3.3, example 1.
Replace structure with:
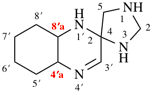
Page 395, P-31.2.3.3.3, example 2.. [corrected 12 May 2021]
For 4,5-dihydro-3H-spiro[1-benzofuran-2,1′-cyclohexan]-2′-ene (PIN)
read 4,5-dihydro-3H-spiro[[1]benzofuran-2,1′-cyclohexan]-2′-ene (PIN)
Page 397, P-31.2.3.3.5.1, example 1.
For 1-(cyclohexa-1,3-dien-1-yl)benzene
read (cyclohexa-1,3-dien-1-yl)benzene
Page 397, P-31.2.3.3.5.1, example 3.
13,16,22,23-tetrahydro-11,21:24,31-terphenyl (PIN)
read 12,15,22,23-tetrahydro-11,21:24,31-terphenyl (PIN)
for 2′,3,3′,6-tetrahydro-1,1′:4′,1′′-terphenyl
read 2,2′,3′,5-tetrahydro-1,1′:4′,1′′-terphenyl
replace structure with:

Page 397, P-31.2.3.3.5.1, example 4. [modified 27 May 2020]
For 11,12,13,14,15,16,21,22,23, 24, 25,26,41,42,43,44,45,46,51,52,53,54,55,56-tetracosahydro-11,21,24, 31,34,41,44,51,54,61-sexiphenyl (PIN)
read 11,12,13,14,15,16,21,22,23,24,25,26,41,42,43,44,45,46,51,52,53,54,55,56-tetracosahydro-11,21:24,31:34,41:44,51:54,61-sexiphenyl (PIN) [remove spaces from name]
For {not 1-(11,21,31,41,51,61, 21,22,23, 24, 25,26-dodecahydro[11,12:42,13-terphenyl]-41-yl)-4-[1,1′-bi(cyclohexan]-4-yl)benzene (substitutive name)}
read {not 1-(21,22,23,24,25,26,31,32,33,34,35,36-dodecahydro[11,21:24,31-terphenyl]-14-yl)-4-([1,1′-bi(cyclohexan)]-4-yl)benzene (substitutive name)} [remove spaces from names]
Page 398, P-31.2.3.3.5.1, example 1 on this page.
For 4-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl)-11,21:24,31-tercyclohexane substitutive name)
read 14-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexane (substitutive name)
for 4-[4-(4′-phenyl[1,1-bi(cyclohexan)]-4-yl)pheny]-1,1′:4′,1′′-tercyclohexane substitutive name)
read 4-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-1,1′:4′,1′′-tercyclohexane (substitutive name)
Page 398, P-31.2.3.3.5.1, example 3 on this page.
For 14,15,24,25,34,35-hexahydro-11H,21H,31H-12,22 :25,33- terazepine (PIN)
read 14,15,24,25,34,35-hexahydro-11H,21H,31H-12,22:25,33-terazepine (PIN) [delete space from name]
for 4,4′,4′′,5,5′,5′′-hexahydro-1H,1′H,1′′H-2,2′:5′ :3′′- tetrazepine
read 4,4′,4′′,5,5′,5′′-hexahydro-1H,1′H,1′′H-2,2′:5′,3′′-tetrazepine [remove space from name]
Page 399, P-31.2.3.3.5.2, example 2 on this page.
For 3a,3′a,4,4′,5,5′,6,6′,7,7′ 7a,7′a-dodecahydro-1H,1′H-2,2′-biindole (PIN)
read 3a,3′a,4,4′,5,5′,6,6′,7,7′,7a,7′a-dodecahydro-1H,1′H-2,2'-biindole (PIN) [replace space by a comma]
Page 400, P-31.2.4.3, example left. [corrected 4 September 2024]
For 2,3,4,5-tetrahydroazocine (PIN)
read (6Z,8Z)-2,3,4,5-tetrahydroazocine (PIN)
Page 401, P-32.1.1, line 1. [corrected 8.11.2023]
For ...formation of preferred IUPAC names, see P-57.1.4.1.
read ...formation of preferred IUPAC names, see P-57.1.1.1.
Page 401, P-32.1.1, example 5. [corrected 6 February 2019]
For (2) 1-methyleth-1-en-1-yl
read (2) 1-methylethen-1-yl
Page 401, P-32.1.1, example 8.
Replace structure to:
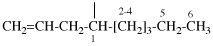
Page 402, P-32.1.1, example 1 on this page.
For (1) hept-1-en-6-yn- -yl (preferred prefix)
read (1) hept-1-en-6-yn-4-yl (preferred prefix)
Page 402, P-32.1.3, example 1. [corrected 11 November 2020]
Replace the structure with:
Page 403, P-32.2.1, example 1. [corrected 19 September 2018]
For 1-azadodeca-1,5,7,9,11-pentaen-6-yl (preferred prefix)
read 1-azacyclododeca-1,5,7,9,11-pentaen-6-yl (preferred prefix)
Page 403, P-32.2.1, example 2. [corrected 19 September 2018]
For 12,13-dihydro-1-aza[13]annulen-4-yl
read 12,13-dihydro-1H-1-aza[13]annulen-4-yl
for 1-azatrideca-2,4,6,8,10-pentaen-4-yl (preferred prefix)
read 1-azacyclotrideca-2,4,6,8,10-pentaen-4-yl (preferred prefix)
Page 405, P-32.3, line 12.
For ...2-phenylethenyl’
read ...2-phenylethen-1-yl.
Page 406, P-32.4, Table 3.2, example 3. [corrected 9 April 2025]
For 2,3-dihydro-1H-isoindole-2-yl
read 1,3-dihydro-2H-isoindole-2-yl
Page 406, P-34.4, Table 3.2 structure 4 on this page.
Replace text with:
isochroman-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers) (preferred prefixes)
isothiochroman-3-yl (S instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isothiochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzothiopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isoselenochroman-3-yl (Se instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isoselenochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzoisoselenopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isotellurochroman-3-yl (Te instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isotellurochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzotelluropyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes) [remove spaces before isomer]
Page 408, P-33.1, example on this page, 2nd compound. (corrected 4 December 2019)
For ethyl (preferred prefix; the prefix ‘-yl describes the loss of one hydrogen atom)
read ethyl (PIN; the prefix ‘-yl’ describes the loss of one hydrogen atom)
Page 408, P-33.1, example on this page, 3rd compound. (corrected 4 December 2019)
For ethan-1-yl-2-ylidene (preferred prefix; the prefix ‘-ylidene’ describes loss the of two hydrogen atom)
read ethan-1-yl-2-ylidene (PIN; the prefix ‘-ylidene’ describes the loss of two hydrogen atoms)
Page 408, P-33.2.1, Table 3.3 (7). [corrected 26 September 2018]
For –CO-NNH2
read –CO-NHNH2
Page 408, P-33.2.1, Table 3.3 (8). [corrected 26 September 2018]
For –(C)O-NNH2
read –(C)O-NHNH2
Page 412, P-33.2.2 (7). [corrected 24 February 2021]
Replace by
Suffixes with –NH2 and =NH groups substituted by an –OH group are named in two ways:
(1) are named as N-hydroxy derivatives of amides or imidic acids;
(2) by modifying the ‘-oic acid’ or ‘-carboxylic acid’ suffix of a systematically named acid to ‘-hydroxamic acid’ or ‘-carbohydroxamic acid’ and ‘-hydroximic acid’ or ‘-carbohydroximic acid’, or the ‘-ic acid’ ending of the retained name of an acid to ‘-hydroxamic acid’ or ‘-hydroximic acid’. The letter ‘o’ is added for euphony between ‘h’ and a preceding consonant.
Method (1) is used for preferred IUPAC names.
Page 413, P-33.3, examples structure 6. [corrected 21 April 2021]
For methanamimiumyl (preferred prefix)
read methanaminiumyl (PIN)
replace structure with:

Page 413, P-33.3, example 8.
For naphthalene-3-yl-1(2H)-ylidene (PIN)
read naphthalen-3-yl-1(2H)-ylidene (PIN)
Page 415, P-34.1.1.2, example. [modified 11 March 2020]
Delete oxaldehyde (PIN, P-66.6.1.2)
for ethanedial
read ethanedial (PIN)
for glyoxal (see P-66.6.1.2; limited substitution allowed: see P-65.1.8.2)
read glyoxal
Page 416, P-34.1.1.5, example 2. [modified 6 February 2019]
For (hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
Page 416, P-34.1.1.5, example 3. [modified 24 October 2018]
For guanidine (special substitution; see P-66.4.1.2.1)
read guanidine (substitution allowed; see P-66.4.1.2.1)
replace the structure with:

Page 417, P-34.1.2, example 1 on this page, the name for the right version. [corrected 6 February 2019]
For (hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
Page 417, P-34.1.2, example 3 on this page.
For carbonimidoyl
read carbonimidic diamide
Page 417, P-34.1.2, example 5 on this page.
For glyoxal (see P-66.6.1.2; limited substitution allowed: see P-65.1.8.2)
read glyoxal (see P-66.6.1.2.1)
Page 417, P-34.1.2, name 5 on this page. [modified 7 August 2019]
Delete this entry
Page 417, P-34.1.2, example 9 on this page.
For benzenzenol
read benzenol
Page 418, P-34.2.1.1 example 2. [corrected 19 September 2018]
For phenylcarbonyl
read benzenecarbonyl
Page 418, P-34.2.1.1 example 3. [corrected 6 February 2019]
For aminocarbonimidoyl
read C-aminocarbonimidoyl
Page 419, P-34.2.1.3, examples 1 on this page. [corrected 26 September 2018]
Replace the structure with:
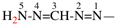
Page 419, P-34.2.1.3, example 2 on this page.
For fornazan-5-yl (preferred prefix) (full substitution; see P-8.3.1.3.5.2)
read formazan-5-yl (preferred prefix) (full substitution; see P-68.3.1.3.5.2)
Page 419, P-34.2.1.3, examples 5 on this page. [corrected 26 September 2018]
Replace the structure with:
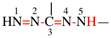
Page 419, P-34.2.1.3, examples 6 on this page. [corrected 10 October 2018]
Replace the structure with:
Page 420, P-34.2.2, example 2. [corrected 25 August 2016]
For anilino (no substitution allowed; see P-62.2.1.1.1)
read anilino (full substitution allowed; see P-62.2.1.1.1)
Page 420, P-34.2.2, example 4 right. [corrected 6 February 2019]
For aminocarbonimidoyl
read C-aminocarbonimidoyl
Page 420, P-34.2.2, example 6. [corrected 6 February 2019]
For carbonamidoyl
read aminocarbonyl
Page 420, P-34.2.2, example 15
For diazany(diazenylmethylidene)
read diazenyl(hydrazinylidene)methyl
Page 420, P-34.2.2, example 16
For diazenyl(hydrazinylidene)methyl
read (diazenylmethylidene)hydrazinyl
Page 421, P-35.0, line 7. [corrected 7 November 2018]
For ...example –S(O2)-OH, –Se(O2)-OH. These prefixes...
read ...example –S(O)2-OH, –Se(O)2-OH. These prefixes...
Page 424, P-35.2.2, example 12 on this page.
Delete this example (=NH). Incorrect and covered by P-35.2.1
Page 426, P-35.3.2, example 5. [corrected 31 October 2018]
For hydroxy(hydrazinylidene)methyl
read hydrazinylidene(hydroxy)methyl
Page 426, P-35.4.1, example 3.
For 4-(chlorophenyl)methoxy (preferred prefix)
read (4-chlorophenyl)methoxy (preferred prefix)
Page 431, P-41, example 2. [corrected 3 June 2020]
For (trimethylazaniumyl)acetate (PIN)
read (N,N-dimethylmethanaminiumyl)acetate (PIN)
Page 431, P-41, example 5. [corrected 3 June 2020]
For (trisulfanyl)silane (preselected name;
read trisulfanylsilane (preselected name;
Page 431, P-41, example 11.
For 1-(methanesulfonyl)-2-(methylsulfanyl)ethane
read 1-(methanesulfinyl)-2-(methylsulfanyl)ethane
for (C > sulfide and sulfone)
read C > sulfide and sulfoxide)
Page 432, P-42.1, example 1.
For –(C)(O)OH -oic acid
read –(C)O-OH -oic acid
Page 434, P-42.4, example 4. [corrected 7 November 2018]
For HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2
read (HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2
Page 434, P-42.4, example 17.
For orthosilicic acid
read silicic acid (formerly orthosilicic acid. See P-67.1.1.1, P-67.1.2.2 and Table IR-8.1, ref. 12.)
Page 434, P-42.4, line 3 from the bottom. [corrected 26 September 2018]
For (HO)SO2-O-[SO2(OH)-O-]nSO2(OH)
read (HO)SO2-O-[SO2-O-]nSO2(OH)
Page 434, P-42.4, line 2 from the bottom. [corrected 26 September 2018]
For (HO)SO-O-[SO(OH)-O-]nSO(OH)
read (HO)SO-O-[SO-O-]nSO(OH)
Page 435, P-42.4, line 1 on this page. [corrected 6 December 2023]
For disulfurous acid
read disulfurous acid (ambiguous see P-67.3.2) p>
Page 436, P-42.5, example 5 on this page.
For hypoiodous IOH
read hypoiodous acid IOH
Page 439, P-43.1, Table 4.3, line 10 on this page.
For sulfonimido(thioperoxoic) OO-acid
read sulfonimido(thioperoxoic) SO-acid
Page 439, P-43.1, Table 4.3, line 14 on this page. [corrected 19 September 2018]
For sulfoimidodithioic acid
read sulfonimidodithioic acid
Page 440, P-43.1, Table 4.4 (1), example 2.
For –(C)OOH peroxoic acid
read –(C)O-OOH peroxoic acid
Page 440, P-43.1, Table 4.4 (1), example 7.
For –CO-SeH selenoic Se-acid
read –(C)O-SeH selenoic Se-acid
Page 440, P-43.1, Table 4.4 (1), last example.
For –C(S)-SH
read –(C)S-SH
Page 441, P-43.1, Table 4.4, section 3, last two examples. [corrected 13 February 2019]
For –C(=NHNH2)-SH
read –C(=NNH2)-SH
for –(C)(=NHNH2)-SH
read –(C)(=NNH2)-SH
Page 442, P-43.1, Table 4.4 (7), line 2.
For Sulfonohydrazonoperoxoic
read Sulfonohydrazonoperoxoic acids
Page 442, P-43.1, Table 4.4 (9), line 4. [corrected 10 October 2018]
For –S(O)(S)-OOH
read –S(S)-OOH
Page 443, P-43.1, Table 4.4 (10), line 2.
For Sulfinoimidoperoxoic acids
read Sulfinimidoperoxoic acids
Page 443, P-43.1, Table 4.4 (10), line 3.
For Sulfinoimidoperoxoic acids modified by replacement with...
read Sulfinimidoperoxoic acids modified by replacement with...
Page 444, P-43.1, Table 4.4 (22), line 3. [corrected 10 October 2018]
For –S(S)(=NH)-NH2
read –S(S)(=NNH2)-NH2
Page 448, P-44.1.1, example 3.
For [not 7-chloro-3-(1-hydroxyethyl)heptan-1-ol; the locant set ‘1,4’ for the principal characteristic groups is lower than ‘2,5’]
read [not 7-chloro-3-(1-hydroxyethyl)heptan-1-ol; there are two of the principal characteristic groups in the parent of the PIN and only one in the other name]
add [not 3-(4-chlorobutyl)pentane-2,5-diol; the locant set ‘1,4’ for the principal characteristic groups is lower than ‘2,5’]
Page 448, P-44.1.1, example 7.
For 7,7-bis[(2-butoxyethoxy)methyl]-3,6,10,13-tetrathiapentadecanedioic acid (PIN)
read 7,7-bis[(2-butoxyethoxy)methyl]-3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
for [not 9-[(2-butoxyethoxy)methyl]-9-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)-11,14-dioxa-3,6-dithiaoctadecanoic acid;
read
[not 9-[(2-butoxyethoxy)methyl]-9-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)-11,14-dioxa-3,6-dithiaoctadecan-1-oic acid;
for [nor 7-[(2-butoxyethoxy)methyl]-7-[2-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)ethyl]-9,12-dioxa-3,6-dithiahexadecanoic acid;
read
[nor 7-[(2-butoxyethoxy)methyl]-7-[2-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)ethyl]-9,12-dioxa-3,6-dithiahexadecan-1-oic acid;
for for ...two of the principal characteristic group in the PIN...
read ...two of the principal characteristic group in the parent of the PIN...
replace the structure with:
Page 448, P-44.1.1, example 8.
For (silanediyldiethane-2,1-diyl)bis(silane)
read [silanediyldi(ethane-2,1-diyl)]bis(silane)
Page 449, P-44.1.1, example 1 on this page. [modified 1 January 2020]
For 4-[4-(pyridin-4-ylmethyl)phenyl]-1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane-14,74-dicarboxylic acid (PIN)
read 4-{4-[(pyridin-4-yl)methyl]phenyl}-1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane-14,74-dicarboxylic acid (PIN)
for [not 4-{[3-(3-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-7(1),3(1,4),5(1,3)-tribenzenaheptaphane-74-carboxylic acid;
there are two of the principal characteristic group in the PIN and only one in the other name]
read [not 4-{[4-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane-74-carboxylic acid;
nor 4-{[3-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane-74-carboxylic acid;
there are two of the principal characteristic group in the PIN and only one in the other names]
replace structure with:
Page 450, P-44.1.2.1, example 4 on this page. [modified 22 May 2019]
For tert-butyl(dimethyl)(oxiran-2-ylmethoxy)silane (PIN)
read tert-butyldi(methyl)(oxiranylmethoxy)silane (PIN)
replace the structure with:
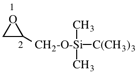
Page 451, P-44.1.2.1, example 1 on this page.
For 2-(phosphinan-2-ylphosphanyl)furan (PIN)
read 2-[(phosphinin-2-yl)phosphanyl]furan (PIN)
Page 451, P-44.1.2.1, example 2 on this page.
For 1-(2H-pyran-3-yl)-2-silolan-2-yl)hydrazine (PIN)
read 1-(2H-pyran-3-yl)-2-(silolan-2-yl)hydrazine (PIN)
Page 451, P-44.1.2.1, example 5. [corrected 19 September 2018]
For 1-[ 5-(2,4,6,8-tetrasilanonan-1-yl)oxan-3-yl]-2,4,6,8-tetraoxaonane (PIN)
read 1-[5-(2,4,6,8-tetrasilanonan-1-yl)oxan-3-yl]-2,4,6,8-tetraoxanonane (PIN) [deleted space in name]
for [O-chain > Si-chain (see P-44.3); senior parent cannot be chosen by P-44.1.2)
read [O-chain > Si-chain (see P-44.3; senior parent cannot be chosen by P-44.1.2)]
replace the structure with:
Page 452, P-44.1.2.2, example 5. [corrected 5 July 2018]
For (2) 1-(pyridin-2-yl)hydrazine
read (2) (pyridin-2-yl)hydrazine
for 1-(pyridin-2-yl)diazane
read (pyridin-2-yl)diazane
Page 452, P-44.1.2.2, example 6. [corrected 3 June 2020]
For 2-hydrazino-4,5-dihydro-1H-imidazole
read 2-diazanyl-4,5-dihydro-1H-imidazole
Page 453, P-44.2.1 (g). [corrected 13 February 2019]
For ...the sequence as given in (c), above.
read ...the sequence: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
Page 454, P-44.2.1.1, example 1 on this page.
For 2-(naphthalen-2-ylmethyl)pyridine (PIN)
read 2-[(naphthalen-2-yl)methyl]pyridine (PIN)
Page 455, P-44.2.1.1, example on this page, explanation line 3. [corrected 18 May 2023]
For ...criterion (f) in P-44.2.1...
read ...criterion (g) in P-44.2.1...
Page 458, P-44.2.1.7 example (3).
For 1-oxadispiro[3.1.36.34]dodecane (PIN)
read 11-oxadispiro[3.1.36.34]dodecane (PIN)
Page 459, P-44.2.1.8 example (2).
For 2,6,8-dioxa-7-stannaspiro[3.5]nonane (PIN)
read 2,6,8-trioxa-7-stannaspiro[3.5]nonane (PIN)
Page 463, P-44.2.2.2.1.3 (b), example.
Replace diagram with:
for II dispiro[[1,3]dioxolane-2,2′-[1]azabicyclo[2.2.2]octane-5′,2′′-oxolane] (PIN)
read I 1′-azadispiro[[1,3]dioxolane-2,2′-bicyclo[2.2.2]octane-5′,2′′-oxolane] (PIN)
for I dispiro[[1]azabicyclo[2.2.2]octane-3,2′-oxolane-4′,2′′-dioxolane] (PIN)
read II 4-azadispiro[bicyclo[2.2.2]octane-2,2′-oxolane-3′,2′′-[1,3]dioxolane] (PIN)
for [first cited component azabicyclo[2.2.2]octane > dioxolane)]
read [first cited component dioxolane > bicyclo[2.2.2]octane]
Page 470, P-44.2.2.2.3.3, examples.
For 5H-[1,3]dioxolo[c][1,2]oxaphosphole (PIN)
read 5H-[1,3]dioxolo[4,5-c][1,2]oxaphosphole (PIN)
for 5H-[1,3]dioxolo[d][1,2]oxaphosphole (PIN)
read 5H-[1,3]dioxolo[4,5-d][1,2]oxaphosphole (PIN)
Page 472, P-44.2.2.2.4.2, example left. [corrected 3 January 2020]
For 1H-3,10a-methanophenanthrene (PIN)
read 1H-3,10a-methanophenanthrene (PIN)
Page 475, P-44.2.2.2.4.9, right example. [corrected 3 January 2020]
For (II) 6,16-methano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
replace the structure with:
 >
>
Page 475, P-44.2.2.2.4.10, right example. [corrected 3 January 2020]
For (I) 6,17-methano-10,13-hexanonaphtho[2,3-c][1]benzazocine (PIN)
read (I) 13,18-methano-6,10-hexanonaphtho[2,3-c][1]benzazocine (PIN)
For (II) 6,16-methano-10,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (II) 13,18-ethano-6,10-heptanonaphtho[2,3-c][1]benzazocine (PIN)
for ...in (I) (atom 21) is fewer than two in (II) (atoms 20 and 21)
read ...in (I) (atom 22) is fewer than two in (II) (atoms 21 and 22)
replace the structure with:
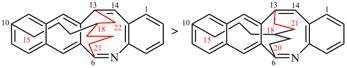
Page 476, P-44.2.2.2.4.12, example. [corrected 3 January 2020]
For (I) 6,16-methano-9,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (I) 13,17-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
for (II) 6,16-methano-10,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
for the locant set ‘9,13’ for the independent bridge in (I) is lower than the locant set ‘10,13’ in (II)
read the locant set ‘6,9’ for the independent bridge in (I) is lower than the locant set ‘6,10’ in (II)
replace the structure with:
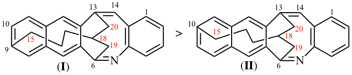
Page 477, P-44.2.2.2.4.13, example. [modified 3 January 2020]
For (I) 6,15-ethano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (I) 13,17-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
For (II) 6,16-ethano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
for the locant set ‘6,15’ for the independent bridge in (I) is lower than the locant set ‘6,16’ in (II)
read the locant set ‘13,17’ for the dependent bridge in (I) is lower than the locant set ‘13,18’ in (II)
replace the structure with:
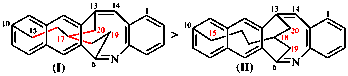
Page 477, P-44.2.2.2.5, line 3.
For ...through P-44.2.2.2.5.5.
read ...through P-44.2.2.2.5.3.
Page 478, P-44.2.2.2.5.2, example. [corrected 13 February 2019]
For [the bridge attachment locant set ‘1,4’ is lower than ‘1,5’]
read [the bridge attachment locant set ‘1,4’ is lower than ‘2,5’]
Page 480, P-44.2.2.2.6.1 (1), first structure. [corrected 31 October 2018]
For 1(4)-pyridina-7(1),3,5(1,4)-tribenzenaheptaphane (PIN)
read 1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
Page 480, P-44.2.2.2.6.1 (1), second structure. [corrected 31 October 2018]
For 1(4)-silina-7(1),3,5(1,4)-tribenzenaheptaphane (PIN)
read 1(4)-silina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
Page 481, P-44.2.2.2.6.1 (4), second structure. [corrected 24 October 2018]
Replace the structure with:
Page 482, P-44.2.2.2.6.2, example (1) second name.
For 1(4)-pyridina-7(2)-silina-3,5(1,4)dibenzenaheptaphane (PIN)
read 1(4)-pyridina-7(2)-silina-3,5(1,4)-dibenzenaheptaphane (PIN)
Page 482, P-44.2.2.2.6.2, example (2), second structure. [corrected 22 May 2019]
Replace the structure with:
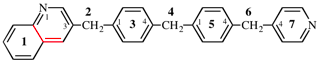
Page 484, P-44.2.2.2.6.4, example 1, second compound
For 1(4),8(4,2)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
read 1(4),8(2,5)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
Page 484, P-44.2.2.2.6.5, example.
Replace first structure with:
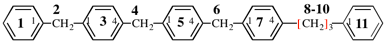
Replace second structure with:
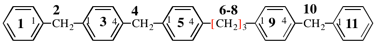
Page 485, P-44.2.2.2.6.6, example first name.
For 1(4),pyridina-7(2,5)-furana-9(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
read 1(4)-pyridina-7(2,5)-furana-9(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
Replace first structure with:
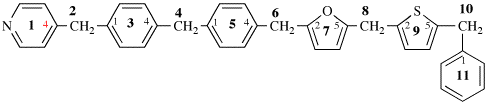
Replace second structure with:
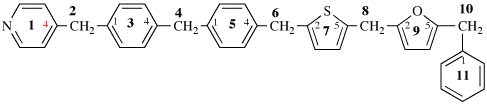
for 1(4)-pyridina-9(2,5)-furana-7(2,5)-thiophena-1,4),11(1)-tribenzenaundecaphane (PIN)
read 1(4)-pyridina-9(2,5)-furana-7(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
Page 485, P-44.2.2.2.6.7, example.
For
1,7(1),3,5(1,3)-dibenzenaheptaphane (PIN)
read 1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN)
for [the locant set ‘1,1,1,3,3’ for amplificant attachment locants in increaing numerical order is lower than ‘1,1,3,4’]
read [the locant set ‘1,1,1,1,3,3’ for amplificant attachment locants in increaing numerical order is lower than ‘1,1,1,1,3,4’]
Page 486, P-44.2.2.2.6.8, example. [corrected 13 February 2019]
Replace the example with:
 |
| 1(4)-pyridina-3(1,3),5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| is senior to |
 |
| 1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane (PIN) |
| [the locant set ‘4,1,3,1,4,1’ for the amplificant locants in order of their citation in the name is lower than ‘4,1,4,1,3,1’] |
Page 487, P-44.2.2.2.6.10, example.
Replace first structure with:
Replace second structure with:
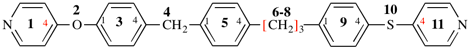
Page 487, P-44.2.2.2.7, example.
Replace structure with:
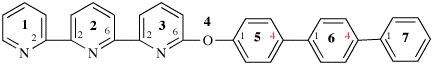
for 4-oxa-1(2),2,3(2,6)-tripyridina-5,6(1,4)7(1)-tribenzenaheptaphane (PIN)
read 4-oxa-1(2),2,3(2,6)-tripyridina-5,6(1,4),7(1)-tribenzenaheptaphane (PIN)
for 16-([11,21:24:31]terphenyl-14-yloxy)-12,22:26,32-terpyridine;
read 16-([11,21:24,31-terphenyl]-14-yloxy)-12,22:26,32-terpyridine;
Page 488, P-44.2.2.2.7, criterion (g).
For ... > Se > Te > P > As...
read ... > Se > Te > N > P > As...
Page 488, P-44.2.2.2.7.1, line 2. [corrected 19 September 2018]
For ... heterocycle [criterion (a) in P-44.2.2.2.7.
read ... heteroatom [criterion (a) in P-44.2.2.2.7.
Page 488, P-44.2.2.2.7.1, example, right structure.
For 1,1′:4,1′′-terphenyl
read 1,1′:4′,1′′-terphenyl
Page 489, P-44.2.2.2.7.4, example, right-hand side.
For 2,2′,2′′-terpyridine
read 2,2′:5′,2′′-terpyridine
Page 489, P-44.2.2.2.7.7, line 2.
For ... > Se > Te > P > As...
read ... > Se > Te > N > P > As...
Page 490, P-44.2.2.2.7.7, example 2.
For 2,2′-bi-3,1,5-benzooxadiarsinine (PIN)
read 2,2′-bi-3,1,5-benzoxadiarsepine (PIN)
Page 491, P-44.3.1, example (2), second structure.
Replace the structure with:
Page 491, P-44.3.2, example (3), first structure.
Replace the structure with:
Page 491, P-44.3.2, example (3), second structure.
Replace the structure with:
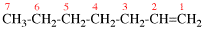
Page 492, P-44.3.2, example (4), second structure.
Replace the structure with:
Page 492, P-44.3.2, example (6).
Replace structure with:
Page 492, P-44.3.2, example (7).
Replace structure with:
Page 494, P-44.4.1.1, example (2).
For [three multiple bonds greater than one]
read [two multiple bonds greater than one]
replace the structures with:
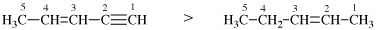
Page 494, P-44.4.1.1 (3), first structure. [corrected 26 September 2018]
Replace the structure with:
.gif)
Page 494, P-44.4.1.1 (3), second structure. [corrected 26 September 2018]
Replace the structure with:
a.gif)
Page 495, P-44.4.1.2 (1), example left. [corrected 4 September 2024]
For cycloicosene (PIN)
read (E)-cycloicosene (PIN)
Page 495, P-44.4.1.2 (2), example left. [corrected 4 September 2024]
For cycloicosa-1,8-diene (PIN)
read (1E,8E)-cycloicosa-1,8-diene (PIN)
Page 495, P-44.4.1.2 (2), example right. [corrected 4 September 2024]
For (1E)-cycloicos-1-en-3-yne (PIN)
read (1E)-cycloicos-1-en-3-yne (PIN)
Page 496, P-44.4.1.2 (5), left structure. [corrected 26 September 2018]
Replace the structure with:
.gif)
Page 496, P-44.4.1.2 (5), right structure. [corrected 26 September 2018]
Replace the structure with:
a.gif)
Page 497, P-44.4.1.3.1, example (1). [corrected 3 June 2020]
For 1,3λ6-thioxolane (PIN)
read 1,3λ6-oxathiolane (PIN)
for 1,3λ4-thioxolane (PIN)
read 1,3λ4-oxathiolane (PIN)
Page 500, P-44.4.1.5, example (1) second structure.
For 1,9-dioxa-4,6-dithia-5-stanna- cycloundecane (PIN)
read 1,9-dioxa-4,6-dithia-5-stannacycloundecane (PIN)
Page 500, P-44.4.1.5, example (3), first structure
For 2-oxa-5-sila-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
read 2-oxa-5-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) [example changed as with silicon P-44.2.2.2.6.10 decides seniority]
replace the structure with:
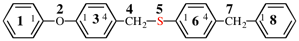
Page 500, P-44.4.1.5, example (3), second structure
For 2-oxa-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane
read 2-oxa-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
Page 502, P-44.4.1.6 (5), second compound. [corrected 31 October 2018]
For 2-oxa-5-selena-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
read 2-oxa-7-thia-5-selena-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
Page 503, P-44.4.1.8, example 1. [corrected 25 August 2016]
For 2H-pyran-2-one (PIN)
read pyridin-2(1H)-one (PIN)
for 4H-pyran-4-one (PIN)
read pyridin-4(1H)-one (PIN) [examples changed as with pyran seniority is decided by P-44.4.1.4]
replace the structures with:
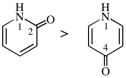
Page 504, P-44.4.1.8, example (2), second compound.
For octane-1,8-diol
read octane-1,8-diol (PIN)
Page 505, 44.4.1.10.1 (1), example left. [corrected 4 September 2024]
For cyclocosa-1,3-dien-5-yne (PIN)
read (1Z,3E)-cyclocosa-1,3-dien-5-yne (PIN)
Page 505, 44.4.1.10.1 (1), example right. [corrected 4 September 2024]
For cyclocosa-1,7-dien-3-yne (PIN)
read (1E,7Z)-cyclocosa-1,7-dien-3-yne (PIN)
Page 506, 44.4.1.10.1 (2), example left. [corrected 4 September 2024]
For cyclocosa-1,3-dien-5-yne (PIN)
read (1Z,3E)-cyclocosa-1,3-dien-5-yne (PIN)
Page 506, 44.4.1.10.1 (2), example right. [corrected 4 September 2024]
For cyclocosa-1,5-dien-3-yne (PIN)
read (1E,5E)-cyclocosa-1,5-dien-3-yne (PIN)
Page 506, P-44.4.1.10.1, example (5).
Replace first structure with:
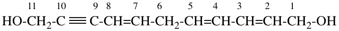
Replace second structure with:
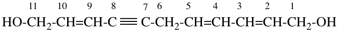
Page 507, P-44.4.1.10.1, example (8).
For 1,13(1),3,6,10(1,4)-pentabenzenatridecaphane-4,11-dien-8-yne (PIN)
read 1,13(1),3(1,2),6,10(1,4)-pentabenzenatridecaphane-4,11-dien-8-yne (PIN)
Page 508, P-44.4.1.10.2, example (2).
Replace first structure with:
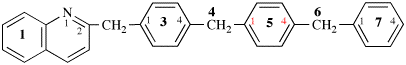
Replace second structure with:
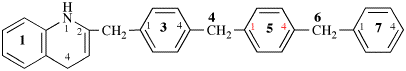
Page 510, P-44.4.1.11.2, example 2 right,
For [1,1-2H]pentane
read [1-2H1]pentane (PIN)
replace the structure with:
Page 510, P-44.4.1.11.3, example 2. [corrected 3 January 2020]
For [81Br]CH2-CH2-CH2-CH2-CH3
read [14C]H3-CH2-CH2-CH2-CH3
for 1-[81Br]bromopentane (PIN)
read [1-14C]pentane (PIN)
for [79Br]CH2-CH2-CH2-CH2-CH3 (PIN)
read [13C]H3-CH2-CH2-CH2-CH3 (PIN)
for 1-[79Br]bromopentane (PIN)
read [1-13C]pentane (PIN)
for [81Br is senior to 79Br]
read [14C is senior to 13C]
Page 511, P-44.4.1.11.5, example.
For [4-2H1,5-13C]pentanoic acid (PIN)
read [5-13C,4-2H1]pentanoic acid (PIN)
Page 512, P-44.4.1.12.1, example (2). [modified 3 June 2020]
For (2Z)-but-2-enoic acid
read (2Z)-2-methylbut-2-enoic acid (PIN)
for (2E)-but-2-enoic acid
read (2E)-2-methylbut-2-enoic acid (PIN)
Page 514, P-44.4.1.12.2, example (1).
For (1R)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1R)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
for (1S)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1S)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
Page 516, P-45.1.1, example on this page.
For 2-chloro-4-(2-chloro-4-carboxyphenoxy)benzoic acid (PIN, substitutive name)
read 4-(4-carboxy-2-chlorophenoxy)-2-chlorobenzoic acid (PIN, substitutive name)
replace structure on this page with:
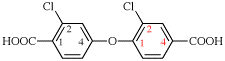
Page 516, P-45.1.2, example 2. [corrected 13 February 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 517, P-45.1.2, last example.
For 1,1′,1′′-({diphenyl[(diphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene (PIN)
read 1,1′,1′′-({[(diphenylmethyl)sulfanyl]diphenylmethoxy}methanetriyl)tribenzene (PIN)
for (not 1,1′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methanediyl)dibenzene
read (not 1,1′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methylene)dibenzene
Page 518, P-45.2.1, example (2).
For [not 3-[(5-chloro-5-cyanophenyl)methyl]benzonitrile; ...
read [not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile; ...
Page 519, P-45.2.1, example (5).
For 2-(naphthalen-2-yl)-13-chloro-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
read 13-chloro-2-(naphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
replace the structure with:
Page 519, P-45.2.1, example (6).
For 1,1-dimethyl-3-[(λ4-thian-3-ylsulfanyl)methyl]- λ4-thiane (PIN)
read 1,1-dimethyl-3-{[(1λ4-thian-3-yl)sulfanyl]methyl}-1λ4-thiane (PIN)
for [not 3-{[(1,1-dimethyl-λ4-thian-2-yl)methyl]sulfanyl}-λ4-thiane;
read [not 3-{[(1,1-dimethyl-1λ4-thian-3-yl)methyl]sulfanyl}-1λ4-thiane;
Page 519, P-45.2.1, example (6). [modified 3 June 2020]
For [not 3-{[(1,1,-dimethyl-λ4-thian-2-yl)methyl]sulfanyl}-λ4-thiane;
read [not 3-{[(1,1-dimethyl-1λ4-thian-3-yl)methyl]sulfanyl}-1λ4-thiane;
Page 519, P-45.2.1, example (7).
For [not 3-isopropylhexane or 3-propan-2-yl;
read [not 3-isopropylhexane or 3-(propan-2-yl)hexane;
Page 519, P-45.2.1, example (8), move to P-45.2.3 and adjust remaining example numbers. [corrected 3 March 2021]
For [not 3-ethyl-6-(2-methylbutyl)-5-propyldecane; the PIN parent chain has more substituents; four simple substituents vs. three (two simple and one compound) substituent]
read [not 6-butyl-3-ethyl-8-methyl-5-propyldecane; both have the set of locants '3,5,6,8' but the PIN has them in the order '5,8,3,6' which is lower than 6,3,8,5]
Page 520, P-45.2.1, example (10), move to P-45.2.2 and adjust the remaining example numbers. [modified 3 March 2021]
For [not 3-chloro-5-(4-hydroxypentan-2-yl)-4-methylnonane-2,8-diol; the PIN parent chain has more substituents; four (three simple and one compound) substituents vs. three (two simple and one compound)]
read [not 7-chloro-5-(3-hydroxybutyl)-4,6-dimethylnonane-2,8-diol: the locant set of the PIN is '3,4,5,6' which is lower than '4,5,6,7']
replace structure with:
Page 520, P-45.2.1, example (12).
For 1-(disilanyldisulfanyl)-2,2,2-trimethyldisilane (PIN)
read 2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
for [not [2-(trimethyldisilanyl)disulfanyl]disilane; the...
read [not [2-(2,2,2-trimethyldisilanyl)disulfan-1-yl]disilane; the...
replace structure with:
Page 521, P-45.2.1, example (14).
For 4-(4-carboxy-3λ6-sulfanylphenoxy)-2-phosphanyl-3λ6-sulfanylbenzoic acid (PIN)
read 4-[4-carboxy-3-(λ6-sulfanyl)phenoxy]-2-phosphanyl-3-(λ6-sulfanyl)benzoic acid (PIN)
for [not 4-(4-carboxy-3-phosphanyl-2λ6-sulfanylphenoxy)-2λ6-sulfanylbenzoic acid)
read [not 4-[4-carboxy-3-phosphanyl-2-(λ6-sulfanyl)phenoxy]-2-(λ6-sulfanyl)benzoic acid;
Page 522, P-45.2.2, example (4).
For 11-bromo-2-(4-chloronaphthalen-2-yl-1(2)-naphthalena-3,5(1,4)tribenzenaheptaphane (PIN)
read 11-bromo-2-(4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4)-tribenzenaheptaphane (PIN)
for [not 2-(1-bromonaphthalen-2-yl)-14-chloro-1(2)-naphthalena-3,5(1,4),7(1)tribenzenaheptaphane (PIN); ...
read [not 2-(1-bromonaphthalen-2-yl)-14-chloro-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN); ...
replace the structure with:
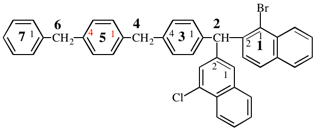
Page 523, P-45.2.2, example (5), move to P-45.2.3 and adjust the remaining example numbers. [corrected 3 March 2021]
For [not 1-ethyl-7-[(5-ethyl-8-propylnaphthalen-2-yl)selenyl]-4-propylnaphthalene; the locant set '1,4,6' in the PIN is lower than '1,4,7']
read [not 4-ethyl-6-[(5-ethyl-8-propylnaphthalen-2-yl)selanyl]-1-propylnaphthalene: both have the locant set '1,4,6' but the PIN set is in the order '1,6,4' which is lower than '4,6,1']
Page 523, P-45.2.2, example (6).
For [not 2-methyl-3-[4-methyl-3-(methylamino)-3-oxopropyl]propanamide; ...
read [not 2-methyl-3-{4-methyl-3-[3-(methylamino)-3-oxopropyl]phenyl}propanamide; ...
Page 524, P-45.2.2, example (9).
For [not 4-(buten-2-yl)-6-methylhepta-1,5-diene, ...
read [not 4-(but-2-en-2-yl)-6-methylhepta-1,5-diene, ...
Page 524, P-45.2.2, example (10), , move to P-45.2.3 and adjust the remaining example numbers. [corrected 3 March 2021]
For [not 1,6-dibromo-3-(1-bromo-2-chloroethyl)-6-chloro-2-iodohexane; the locant set '1,1,4,5,6' in the PIN is lower than '1,2,3,6,6']
read [not 1,6-dibromo-4-(1-bromo-2-chloroethyl)-1-chloro-5-iodohexane; both have the locant set '1,1,4,5,6' but the PIN set is in the order '1,5,4,1,6' which is lower than '1,6,4,1,5']
Page 524, P-45.2.2, example (11).
For 5-bromo-3-(3-nitro-1λ5-phosphanylpropyl)-4λ5-phosphanylhexanoic acid (PIN)
read 5-bromo-3-[3-nitro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid (PIN)
for [not 3-(2-bromo-1λ5-phosphanylpropyl)-6-nitro-4λ5-phosphanylhexanoic acid,
read [not 3-[2-bromo-1-(λ5-phosphanyl)propyl]-6-nitro-4-(λ5-phosphanyl)hexanoic acid
Page 524, P-45.2.2, example (12).
For [not 4-[(4-carboxy-3-(λ5-phosphanyl)phenoxy]-3-phosphanylbenzoic acid;
read [not 4-[4-carboxy-3-(λ5-phosphanyl)phenoxy]-3-phosphanylbenzoic acid;
Page 526, P-45.2.3, example (3). [corrected 3 January 2020]
For not 2-ethyl-7-[(7-ethyl-8-propylnaphthalen-2-yl)oxy]-1-propylnaphthalene
read not 2-ethyl-7-[(8-ethyl-7-propylnaphthalen-2-yl)oxy]-1-propylnaphthalene
replace the structure with:
.gif)
Page 526-527, P-45.2.3, after example (6) add examples (7)-(12) which are transferred from P-45.5.1. For a PDF (348 KB) of the revised P-45.2.3 and P-45.5.1 click here
Page 527, P-45.2.3, example (5).
Replace structure with:
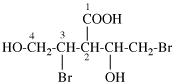
Page 528, P-45.3.1, line 2/3. [corrected 3 January 2020]
For ...cited as prefixes.
read ...cited as prefixes and directly connected to the parent structure.
Page 528, P-45.3.1, example 2. [modified 23 December 2020]
For 4-(2λ5-diphosphan-1-yl)-2-[2-(diphosphan-1-yl)ethyl]butanenitrile (PIN)
read 4-(2λ5-diphosphan-1-yl)-2-(2-diphosphanylethyl)butanenitrile (PIN)
for [not 4-(diphosphan-1-yl)-2-[2-(2λ5-diphosphan-1-yl)ethyl]butanenitrile;
read [not 4-diphosphanyl-2-[2-(2λ5-diphosphan-1-yl)ethyl]butanenitrile;
Page 529, P-45.3.2, example 1. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 529, P-45.3.2, example 2 and explanation. [corrected 3 January 2020]
Delete as decided by P-45.2.3.
Page 529, P-45.3.2, new example. [corrected 3 June 2020]
Add
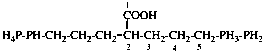
5-(1λ5-diphospan-1-yl)-2-[3-(2λ5-diphospan-1-yl)propyl]pentanoic acid (PIN)
[not 5-(2λ5-diphospan-1-yl)-2-[3-(1λ5-diphospan-1-yl)propyl]pentanoic acid]
The locant ‘1λ5’ for the nonstandard bonding number directly bonded to the parent structure is lower than ‘2λ5’.
Page 529, P-45.4, title. [corrected 3 January 2020]
For CRITERIA RELATED ONLY TO ISOTOPIC MODIFICATION, OTHER CRITERIA BEING EQUAL
read CRITERIA RELATED ONLY TO ISOTOPIC MODIFICATION OF SUBSTITUENTS
Page 529, P-45.4.1. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.1 and inappropriate in P-45.4.
Page 529, P-45.4.1 replacement structure. [modified 3 June 2020]
For 2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane
read 2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane (PIN)
for [not 2-(81)bromo-1-[(2-bromopentyl)oxy]pentane]
read [not 2-(81Br)bromo-1-[(2-bromopentyl)oxy]pentane]
Page 530, P-45.4.2. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.2 and inappropriate in P-45.4.
Page 530, P-45.4.3. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.3 and inappropriate in P-45.4.
Page 530, P-45.4.4, title. [corrected 3 January 2020]
For P-45.4.4
read P-45.4.1
Page 530, P-45.4.1 (formerly P-45.4.4), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 530, P-45.4.1 (formerly P-45.4.4) new example. [corrected 3 January 2020]
Add
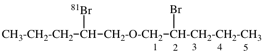
2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane (PIN)
[not 2-(81Br)bromo-1-[(2-bromopentyl)oxy]pentane]
The isotopically modified substituent for the PIN is attached at ‘1’ which is lower than ‘2’.
Page 531, P-45.4.5, title. [corrected 3 January 2020]
For P-45.4.5
read P-45.4.2
Page 531, P-45.4.2 (formerly P-45.4.5), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 531, P-45.4.2 (formerly P-45.4.5), new example. [modified 30 March 2022]
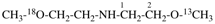
For 2-[(13C)methyloxy]-N-{2-[methyl(18O)oxy]ethyl}ethan-1-amine (PIN)
read 2-(13C)methoxy-N-[2-(18O)methoxyethyl]ethan-1-amine (PIN)
for [not 2-[methyl(18O)oxy]-N-{2-[(13C)methyloxy]ethyl}ethan-1-amine]
read [not 2-(18O)methoxy-N-[2-(13C)methoxyethyl]ethan-1-amine]
18O > 13C; the 18O isotopically modified substituent for the PIN is attached at ‘N’ which is lower than ‘2’.
Page 531, P-45.4.6, title. [corrected 3 January 2020]
For P-45.4.6
read P-45.4.3
Page 531, P-45.4.3 (formerly P-45.4.6), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 531, P-45.4.3 (formerly P-45.4.6), new example. [modified 30 March 2022]
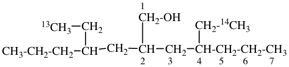
For 4-([2-13C]ethyl)-2-[2-([2-14C]ethyl)pentyl]heptan-1-ol (PIN)
read 4-[2-13C]ethyl-2-(2-[2-14C]ethylpentyl)heptan-1-ol (PIN)
for [not 4-([2-14C]ethyl)-2-[2-([2-13C]ethyl)pentyl]heptan-1-ol
read [not 4-[2-14C]ethyl-2-(2-[2-13C]ethylpentyl)heptan-1-ol
14C > 13C; the 14C isotopically modified substituent for the PIN is attached at ‘2’ which is lower than ‘4’
Page 531-535, P-45.5.1, examples (4), (5), (7)-(9) and (11) are transferred to P-45.2.3.
Page 531, P-45.5.1. title. [corrected 3 January 2020]
For P-45.5.1
read P-45.5
Page 532, P-45.5.1, example (1).
For but ‘bromo’. in the PIN is earlier alphabetically than ‘dibromo’.
read in both names the locant set is ‘1,2,4’, and the locants appear in the name in the same order set ‘1,4,2’ so
no decision can be made by P-45.2.2 or P-45.2.3; but but ‘bromo’. in the PIN is earlier alphabetically than ‘dibromo’.
Page 532, P-45.5.1, example (2).
For 4-bromo-N-(2,4-dibromophenyl)-2-nitroaniline] (PIN)
read 2-bromo-4-chloro-N-(2,4-dibromophenyl)aniline (PIN)
for [not 2,4-dibromo-N-(4-bromo-2-nitrophenyl)aniline;
read [not 2,4-dibromo-N-(2-bromo-4-chlorophenyl)aniline;
replace structure with:
Page 532, P-45.5.1, example (3).
For 14-bromo-13-chloro-2-(3,4-dibromonaphthalen-1-yl)-1(2)naphthalene-3,5(1,4),7(1)tribenzenaheptaphane (PIN)
read 13-bromo-14-chloro-2-(3,4-dibromonaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
for [not 13,14-dibromo- 2-(4-bromo-3-chloronaphthalen-2-yl)-1(2)naphthalene- 3,5(1,4),7(1)-tribenzenaheptaphane;
read [not 13,14-dibromo-2-(3-bromo-4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane; [remove spaces from name]
replace structure with:
Page 533, P-45.5.1, example (4). Move to P-45.2.3 as example (7).
For 4-bromo-2-(2-chloroethyl)butan-1-ol (PIN)
read 4-bromo-2-(2-chloroethyl)butan-1-ol
for 2-(2-bromoethyl)-4-chlorobutan-1-ol
read 2-(2-bromoethyl)-4-chlorobutan-1-ol (PIN)
for (‘bromo-chloro’ in the PIN is earlier alphabetically than ‘bromo-ethyl’)
read (the locant sets are the same in both names, i.e., ‘2,4’ but in the order of appearance in the name, the locant set ‘2,4’ in the PIN is lower than ‘4,2’)
Page 533, P-45.5.1, example (5). Move to P-45.2.3 as example (8).
For (‘bromo’ in the PIN is earlier alphabetically than ‘dibromo’)
read (the locant sets are the same in both names, i.e., ‘1,1,5,6’ but in their order of appearance in the name, the locant set ‘1,5,1,6’ is lower than ‘1,6,1,5’)
Page 533, P-45.5.1, example (6).
Renumber as example (4)
Page 534, P-45.5.1, example (7). Move to P-45.2.3 as example (9).
For (‘ethyl....ethyl’ in the PIN name is earlier alphabetically than ‘ethyl....methyl’]
read (the locant sets are the same in both names, i.e. ‘6,7,8’ but in their order of appearance in the name, the locant set ‘7,6,8’ is lower than ‘8,7,6’)
Page 534, P-45.5.1, example (8). Move to P-45.2.3 as example (10).
For 3-{3-[3-(ethylamino)-3-oxopropyl]-4-methylphenyl}-N-methylpropanamide (PIN)
read N-methyl-3-{4-methyl-3-[3-oxo-3-(propylamino)propyl]phenyl}propanamide (PIN)
for [not N-ethyl-3-{2-methyl-5-[3-(methylamino)-3- oxopropyl]phenyl}propanamide;
oxopropyl]phenyl}propanamide; ‘ethylamino’ in the PIN is earlier alphabetically than ‘ethyl....methyl’]
read [not 3-{2-methyl-5-[3-(methylamino)-3-oxopropyl]phenyl}-N-propylpropanamide;
(the locant sets are the same in both names, i.e. ‘N,3’ but in their order of appearance in the name, the locant set ‘N,3’ in the PIN is lower than ‘3,N’)
replace structure with:
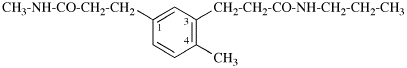
Page 534, P-45.5.1, example (9). Move to P-45.2.3 as example (11).
For 5-bromo-3-[2-chloro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid (PIN)
read [not 5-bromo-3-[2-chloro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid; [move to after corrected PIN]
for [not 3-[2-bromo-1-(λ5-phosphanyl)propyl]-5-chloro-4-(λ5-phosphanyl)hexanoic acid;
read
3-[2-bromo-1-(λ5-phosphanyl)propyl]-5-chloro-4-(λ5-phosphanyl)hexanoic acid (PIN)
for ‘bromo-chloro’ is lower alphanumerically than ‘bromo-phosphanyl’]
read the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order of appearance in the name, the locant set ‘3,5,4’ in the PIN is lower than ‘5,3,4’]
retain structure:
Page 535, P-45.5.1, example (10).
Renumber as example (5).
Page 535, P-45.5.1, example (11). Move to P-45.2.3 as example (12).
For 4-(81Br)bromo-5-bromo-3-[1-(81Br)bromo-2-chloropropyl]hexanoic acid (PIN)
read [not 4-(81Br)bromo-5-bromo-3-[1-(81Br)bromo-2-chloropropyl]hexanoic acid; [move to after corrected PIN]
for [not 4-(81Br)bromo-3-[1-(81Br)bromo-2-bromopropyl]-5-chorohexanoic acid
read 4-(81Br)bromo-3-[1-(81Br)bromo-2-bromopropyl]-5-chlorohexanoic acid (PIN)
for ‘bromo....bromo....bromo....chloro’ is lower than ‘bromo’....bromo....bromo....propyl]
read the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order of appearance in the name, the locant set ‘4,3,5’ in the PIN is lower than ‘4,5,3’]
Page 535, P-45.5.2. [corrected 3 January 2020]
Delete as decided by P-45.4.3 (formerly P-45.4.6).
Page 536, P-45.6.2, lines 2/3. [corrected 3 January 2020]
For ...and P-44.4.1.10 is not...
read ...and P-44.4.1.12) is not...
Page 536, P-45.6.2, line 5.
For The names with and without stereodescriptors are the same, i.e. butane.
read The names with and without stereodescriptors are the same, i.e. butene.
Page 536, P-45.6.2, example 1.
For (1E,1′E)-1,1′-sulfanediyldi[(1E)-cyclooct-1-ene] (PIN)
read (1E,1′E)-1,1′-sulfanediyldi(cyclooct-1-ene) (PIN)
Page 536, P-45.6.2, the paragraph 2, line 2.
For ... dependent as shown in P-47.1.2 and P-47.2.2 below. Two...
read ... dependent as shown below. Two...
Page 537, P-45.6.2, example (1) cont'd.
For (1Z,3S)-3-{2-[(1E,3R)-cyclooct-1-en-3-yl]ethyl}cyclooct-1-ene (PIN)
read (1Z,3S)-3-{2-[(1R,2E)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene (PIN)
for (1-cis,3S)-3-{2-[(1-trans,3R)-cyclooct-1-en-3-yl]ethyl}cyclooct-1-ene
read (1-cis,3S)-3-{2-[(1R,2-trans)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene
Page 537, P-45.6.2, example 2.
For 1-[(2R)-butan-2-yl-]-4-({4-[(2S)-butan-2-yl]phenyl}sulfanyl)benzene (PIN)
read
1-[(2R)-butan-2-yl]-4-({4-[(2S)-butan-2-yl]phenyl}sulfanyl)benzene (PIN)
for
1-[(2S)-butan-2-yl-]-4-({4-[(2R)-butan-2-yl]phenyl}sulfanyl)benzene
read
1-[(2S)-butan-2-yl]-4-({4-[(2R)-butan-2-yl]phenyl}sulfanyl)benzene
replace the second structure with:
Page 537, P-45.6.2, example 3.
For 1,1′-oxybis[bis(3,4-(1-chloroethyl)benzene]
read 1,1′-oxybis[3,4-bis(1-chloroethyl)benzene]
Page 538, P-45.6.2, Example 3, second compound on this page. [modified 3 January 2020]
For 1,2-bis[(1S)-1-chloroethyl]-4-{4-[(1S)-1-chloroethyl]-2-[(1R)-1-chloroethyl]phenoxy}benzene (PIN)
read 1,2-bis[(1S)-1-chloroethyl]-4-{3-[(1R)-1-chloroethyl]-4-[(1S)-1-chloroethyl]phenoxy}benzene (PIN)
for ...; ‘bis.....chloroethyl....chloroethyl’ is lower alphabetically than ‘bis.....chloroethylphenoxy’]
read ...; ‘1,2,4’ is senior to ‘4,2,1’ (see P-45.2.3)]
Page 538, P-45.6.3, example.
For 1-[(R)-(1-bromoethyl)-1-[(S)-(1-bromoethyl)}cyclopentane
read 1-[(1R)-1-bromoethyl]-1-[(1S)-1-bromoethyl]cyclopentane
for 1-[(S)-(1-bromoethyl)-1-[(R)-(1-bromoethyl)}cyclopentane
read 1-[(1S)-1-bromoethyl]-1-[(1R)-1-bromoethyl]cyclopentane
Page 539, P-45.6.4. [corrected 3 January 2020]
Delete as decided by P-44.4.1.12.
Page 544, P-46.1.5, example 1.
For 5-{[(methoxymethyl)sulfanyl]]methyl}-4,9-dioxa-2,7λ4-dithiadecan-1-yl (preferred prefix)
read 5-{[(methoxymethyl)sulfanyl]methyl}-4,9-dioxa-2,7λ4-dithiadecan-1-yl (preferred prefix)
Page 544, P-46.1.5, example 2.
For 5-({[(methylsulfanyl)methyl]-λ6-sulfanyl]}methyl)-4-oxa-2λ4,7λ4,9λ4-trithiadecan-1-yl (preferred prefix)
read 5-({[(methylsulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ4-trithiadecan-1-yl (preferred prefix)
for [not 5-({[(methyl-λ4-sulfanyl)methyl]-λ4-sulfanyl]}methyl)-4-oxa-2λ4,7λ6,9-trithiadecan-1-yl;
read [not 5-({[(methyl-λ4-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9-trithiadecan-1-yl;
Page 544, P-46.1.5, example 3.
For 5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl]}methyl)-4-oxa-2λ4,7λ6 ,9λ6 -trithiadecan-1-yl (preferred prefix)
read 5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9λ6-trithiadecan-1-yl (preferred prefix) [deleted spaces in name]
for [not 5-({[(methyl-λ6-sulfanyl)methyl]-λ6-sulfanyl]}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
read [not 5-({[(methyl-λ6-sulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
Page 546, P-46.1.10, example 1.
For 5-({[2-(methyl-λ4-sulfanyl)ethyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiaundecan-1-yl (preferred prefix)
read 5-({[2-(methyl-λ6-sulfanyl)ethyl]sulfanyl}methyl)-4-oxa-2λ4,7λ4,10-trithiaundecan-1-yl (preferred prefix)
for [not 5-({[(ethyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ4,10λ4-trithiadecan-1-yl; the locant set for the λ4 atoms ‘2,7,9’ is lower than ‘2,7,10’]
read [not 5-({[(methylsulfanyl)ethyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7,10λ6-trithiaundecan-1-yl; the locant set for the nonstandard bonding atoms ‘2,7’ is lower than ‘2,10’]
Replace the structure with:
Page 547, P-46.1.11, example 1, right version.
For [not-(hydroxymethyl)ethyl]
read [not 1-(hydroxymethyl)ethyl]
Page 547, P-46.1.11, example 2, left version.
For [not 7-chloro-5-(1,2-dichloropropyl)]octan-2-yl; a complex substituent group)
read [not 7-chloro-5-(1,2-dichloropropyl)octan-2-yl; a complex substituent group]
replace structure with:
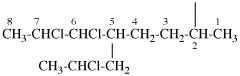
Page 547, P-46.1.11, example 3 left version.
For 2λ5,6-bis(phosphanyl)heptan-4-yl
read (2) 2-(λ5-phosphanyl)-6-phosphanylheptan-4-yl
Page 547, P-46.1.11, example 3 right version.
For 3-(λ5-phosphanyl)-1-(2-phosphanylpropyl)butyl
read (1) 3-(λ5-phosphanyl)-1-(2-phosphanylpropyl)butyl
replace structure with:
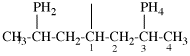
Page 547, P-46.1.11, example 4 left version.
For 2,3,5λ5-tris(phosphanyl)heptan-4-yl
read (2) 5-(λ5-phosphanyl)-2,3-bis(phosphanyl)heptan-4-yl
Page 547, P-46.1.11, example 4 right version.
For 2,3-bis(phosphanyl)-1-[1-(λ5-phosphanyl)propyl]butyl
read (1) 2,3-bis(phosphanyl)-1-[1-(λ5-phosphanyl)propyl]butyl
replace the structure with:
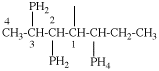
Page 549, P-46.1.12, left example on this page. [corrected 3 January 2020]
Replace the structure with:
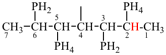
Page 549, P-46.1.12, left example on this page method (2).
For (2) 2λ5,3,5λ5,6-tetakis(phosphanyl)heptan-4-yl
read (2) 2,5-bis(λ5-phosphanyl)-3,6-bis(phosphanyl)heptan-4-yl
for [not 2,5-bis(phosphanyl)-3,6-bis(λ5-phosphanyl)heptan-4-yl;
read [not 3,6-bis(λ5-phosphanyl)-2,5-bis(phosphanyl)heptan-4-yl;
Page 549, P-46.1.12, right example on this page. [corrected 3 January 2020]
Replace the structure with:
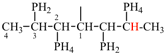 >
>
Page 549, P-46.1.12, right example on this page method (1).
For (1) 2λ5,3-bis(phosphanyl)-1-[1,2λ5-bis(phosphanyl)propyl]butyl
read (1) 2-(λ5-phosphanyl)-3-phosphanyl-1-[2-(λ5-phosphanyl)-1-phosphanylpropyl]butyl
Page 549, P-46.1.13, example 1 right. [corrected 3 January 2020]
For [not 1-(bromoethyl)-2-chloropropyl;
read [not 1-(1-bromoethyl)-2-chloropropyl;
Page 549, P-46.2.1, example 1.
For (1) 3-methyl-4-[2H]butyl (preferred prefix)
read (1) 3-methyl[4-2H1]butyl (preferred prefix)
Page 550, P-46.2.1, example 1 on this page.
For (2) 1-(1-18O)hydroxy-3-hydroxypropan-2-yl (preferred prefix)
read (2) 1-(18O)hydroxy-3-hydroxypropan-2-yl (preferred prefix)
Page 550, P-46.2.2, example.
For (1) 3-[2H]methyl-4-[14C]butyl (preferred prefix)
read (1) 3-[2H1]methyl[4-14C]butyl (preferred prefix)
Page 551, P-46.3.1, example on this page. [modified 22 January 2020]
Add (2) [(2Z,4R,5E)-4-methylhepta-2,5-dien-4-yl]siline (PIN)
(1) {(1R,2Z)-1-[(1E)-prop-1-en-1-yl]-1-methylbut-2-en-1-yl}siline
Replace the structures with:
Page 551, P-46.3.2, example 1 method (1).
For (4R)-4-chloro-1-[(3S)-3-chorobutyl]-1-methylpentyl
read (4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl
Page 551, P-46.3.2, Note lines 4/5. [corrected 3 January 2020]
For ...and a ‘s’ configuration...
read ...and an ‘s’ configuration...
Page 551, P-46.3.2, example 2. [modified 22 January 2020]
Add (2) [(2R,5s,8S)-2,8-dichloro-5-methylnonan-5-yl]trimethylsilane (PIN)
(1) {(1s,4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl}trimethylsilane
Replace the structures with:
Page 553, P-51.1.1, example. [corrected 3 June 2020]
For 1,2-di(propan-2-ylidene)hydrazine (PIN)
read di(propan-2-ylidene)hydrazine (PIN)
Page 554, p-51.1.5, example. [corrected 3 June 2020]
For 2-[2-(carboxymethoxy)ethoxy]acetic acid (substitutive name)
read [2-(carboxymethoxy)ethoxy]acetic acid (substitutive name)
Page 555, P-51.2.1, example 1.
For N,N-dimethylmethanamine oxide (PIN...
read N,N-dimethylmethanamine N-oxide (PIN...
Page 555, P-51.2.1, example 1. [corrected 3 June 2020]
Add (N,N-dimethylmethanaminiumyl)oxidanide
Page 555, P-51.2.1, example 2. [modified 11 November 2020]
For N-chloromethanimine oxide (PIN...
read N-chloromethanimine N-oxide (PIN...
Add (N-chloromethaniminiumyl)oxidanide
Page 555, P-51.2.1, example 2. [corrected 3 June 2020]
Add (N,N-dimethylmethaniminiumyl)oxidanide
Page 555, P-51.2.1, example 3. [corrected 8.11.2023]
For acetyl chloride (PIN; P-65.5.1.1)
read acetyl chloride (PIN; P-65.5.1)
Page 555, P-51.2.1, example 5.
Replace structure with:
CH3-CH2-CO-CN
Page 556, P-51.2.2, example 3. [corrected 3 June 2020]
For 1,2-di(propan-2-ylidene)hydrazine (PIN)
read di(propan-2-ylidene)hydrazine (PIN)
Page 556, P-51.2.2, example 4. [corrected 24 July 2019]
For N-propylidenehydroxylamine (PIN)
read N-propylidenehydroxylamine
add N-hydroxypropan-1-imine (PIN)
Page 558, P-51.3.1, example 1 on this page. [corrected 29 January 2020]
Replace the structures with:
Page 558, P-51.3.1, example 2 on this page.
For 3,3′-[oxybis[(1-chloroethane-2,1-diyl)]oxy]di(propan-1-ol) (PIN...
read 3,3′-{oxybis[(1-chloroethane-2,1-diyl)oxy]}di(propan-1-ol) (PIN...
Page 558, P-51.3.1, example 6 on this page (example 7 of section). [corrected 9 April 2025]
For 2,2′-{ethane-1,2-diylbis[(oxyethane-2,1-diyl)oxy]diacetic acid (a multiplicative name)
read 2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxy)]diacetic acid (a multiplicative name)
Page 559, P-51.3.1, last example. [corrected 7 November 2018]
For 2,2′-{[ethane-1,2-diylbis(oxyethane-2,1-diyloxyethane-2,1-diyl)}dibenzoic acid
read 2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxyethane-2,1-diyl)]dibenzoic acid
Page 560, P-51.3.2.1, example 1.
For 4,4′,4′′-ethane-1,1,2-triyltribenzoic acid (PIN)
read 4,4′,4′′-(ethane-1,1,2-triyl)tribenzoic acid (PIN)
Page 561, P-51.3.2.1, example 1 on this page. [corrected 24 October 2018]
For [not {(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methanediyl}dibenzene];
read [not 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene];
for [not ({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
read [not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
for PIN is lower alphanumerically (‘diphenyltriphenylmethyl’) is lower...
read PIN is lower alphabetically (‘diphenyltriphenylmethoxy’) is lower...
replace the structure with:
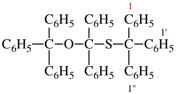
Page 561, P-51.3.2.1, last example.
Replace structure by:
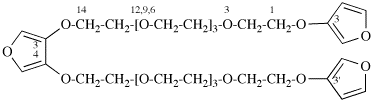
Page 562, P-51.3.2.2, example 1. [modified 29 January 2020]
For 1,1′-oxybis(3,1-phenoxybenzene) (multiplicative name)
read 1,1′-[oxybis(3,1-phenyleneoxy)]dibenzene (multiplicative name)
Delete [not 1,1′-oxybis(3,1-phenyleneoxy)dibenzene (multiplicative name)
Page 562, P-51.3.2.2, example 3. [modified 7 November 2018]
For [not 3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxyfuran-4,3-diyloxyethane-2,1-diyloxy]difuran (a multiplicative name)]
read [not 3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxyfuran-4,3-diyloxyethane-2,1-diyloxy)]difuran (a multiplicative name)]
for 2,5,7,10,12,15,17,20-octaoxa-1,21(3),6,11,16,21(3,4)-pentafuranahenicosaphane (PIN, a phane name see P-51.4)
read 2,5,7,10,12,15,17,20-octaoxa-1,21(3),6,11,16(3,4)-pentafuranahenicosaphane (PIN, a phane name see P-51.4, P-52.2.5)
Page 563, P-51.3.2.3, example.
For ...; not 1,1′-carbonylbis(diazene-2,1-diyl)]dibenzene; ...
read ...; not 1,1′-[carbonylbis(diazenediyl)]dibenzene; ...
Page 563, P-51.3.3, line 1.
For ...as defined in P-53.3.1, above,...
read ...as defined in P-51.3.1, above,...
Page 563, P-51.3.3, example 2.
For [not (disilanylperoxy)methyl]disilane;
read [not [(disilanylperoxy)methyl]disilane;
Page 564, P-51.4.1.2, example 3. [corrected 29 January 2020]
For 3,6,9,12-tetraoxatetradecane-1,14-dioic acid [PIN,
read 3,6,9,12-tetraoxatetradecanedioic acid [PIN,
for ...a multiplicative name]
read ...(a multiplicative name)
for ...a substitutive name]
read ...(a substitutive name)
Page 565, P-51.4.1.2, last example on this page.
For (not 3,4,6-trioxa-5-silanonanan-7-one;...
read (not 2,2,5,5-tetramethyl-3,4,6-trioxa-5-silanonan-7-one;...
Page 568, P-51.4.2.1, example 1.
Replace structure with:
Page 568, P-51.4.2.1, example 2. [modified 29 January 2020]
Delete this correction. These are organic substitutive names and chloro is the substituent name (RB page 138). The error is on page 1044, P-69.4, example 1
Page 569, P-51.4.2.4, example. [modified 29 January 2020]
Replace the structures with:
for 3a1-azaphenalene (PIN)
read 1,3a1,4 -triazaphenalene (PIN)
for 9b-azaphenalene (PIN)
read [not 1,4,9b-triazaphenalene; see P-25.3.3.3]
Page 569, P-51.4.2.6, example 1.
For 13,23,34-trioxa-11,21,26,31-tercycloundecane (PIN;...
read 13,23,34-trioxa-11,21:26,31-tercycloundecane (PIN;...
for 3,3′,4′-trioxa-1,1′;6′,1′′-tercycloundecane
read 3,3′,4′′-trioxa-1,1′:6′,1′′-tercycloundecane
for 13,23,28,33-ter-1-oxacycloundecane
read 13,23:29,34-ter-1-oxacycloundecane
Page 570, P-51.4.2.6, example on this page.
For 23-thia-12,22,26,32-terbicyclo[2.2.1]heptane (PIN;
read 23-thia-12,22:26,32-terbicyclo[2.2.1]heptane (PIN;
for 3′-thia-1,1′;6′,1′′-terbicyclo[2.2.1]heptane
read 3′-thia-2,2′:6′,2′′-terbicyclo[2.2.1]heptane
Page 570, P-51.5, example 1.
For 2,2′-naphthalene-2,3-diyldiacetic acid (PIN)
read 2,2′-(naphthalene-2,3-diyl)diacetic acid (PIN)
for naphthalene-1,3-diacetic acid
read naphthalene-2,3-diacetic acid
Page 571, P-51.5, example 1 on this page.
For benzene-1,3,5-triyltriacetic acid (PIN)
read 2,2′,2′′-(benzene-1,3,5-triyl)triacetic acid (PIN)
Page 572, P-52.1.3, lines 2/3. [corrected 3 June 2020]
For ...except where 'b' is carbon or nitrogen, are described...
read ...except carbon or halogen, are described...
Page 572, P-52.1.3, example 1. [corrected 20 February 2019]
For [not (distannyloxy)stannane]
read [not bis(stannyloxy)stannane]
Page 572, P-52.1.3, example 4.
For (not tristannazane)
read (not trisilazane)
Page 572, P-52.1.4, example 4.
For SH5
read SH6
Page 573, P-52.1.5.1, example 1. [corrected 27 May 2020]
Delete pentazolane
Page 573, P-52.1.5.1, example 3. [corrected 11 November 2020]
Replace the structure with:
 >
>
Page 574, P-52.1.5.2, line 2 on this page. [corrected 10 October 2018]
For ...alternating heteroatoms, i.e., [aba]n, are...
read ...alternating heteroatoms, i.e., [ab]n, are...
Page 575, P-52.1.6.2, example 2 . [corrected 19 September 2018]
For 2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilabicyclo[3.3.1.13,7]decane
read 2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilatricyclo[3.3.1.13,7]decane
Page 576, P-52.1.7, example 1 on this page. [modified 11 November 2020]
For 1H,5H-pentarsolopentarsole (preselected name)
read 1H,4H-pentarsolopentarsole (preselected name)
for 1H,5H-octaarsapentalene
read 1H,4H-octaarsapentalene (numbering shown)
replace the structure with:

Page 576, P-52.1.7, example 2. [modified 11 November 2020]
For [1,3,5,2,4,6]triazatriborino[1,2-a][1,3,5,2,4,6]triazatriborine (preselected name)
read [1,3,5,2,4,6]triazatriborinino[1,2-a][1,3,5,2,4,6]triazatriborinine (preselected name)
replace the structure with:
Page 578, P-52.2.4.1, lines 1/2. [corrected 29 January 2020]
For ...having at least two five-membered rings. This...
read ...having at least two rings of at least five or more members. This...
Page 579, P-52.2.4.2, example 1. [corrected 20 February 2019]
For dibenzo[g, p] [1,3,6,9,12,15,18]heptaoxacycloicosine (PIN; see P-25.3.6.1)
read 9H-dibenzo[g, p] [1,3,6,9,12,15,18]heptaoxacycloicosine (PIN; see P-25.3.6.1)
Page 581, P-52.2.4.4.2, last line.
For ...here in P-52.2.4.4.
read ...here in P-52.2.4.4.2.1.
Page 582, P-52.2.4.4.2.2, example 2. [corrected 20 February 2019]
For 4H-9,2b-(epoxymethano)-2-oxacycloocta[cd]pentalene
read 4H-9,2a1-(epoxymethano)-2-oxacycloocta[cd]pentalene
Page 583, P-52.2.4.4.2.2, example 1 on this page. [corrected 20 February 2019]
For 2H-4,7,12-trioxa-1-thia-5,9b-[1,2]epicyclopentacyclopenta[2′,3′:6,7]cyclohepta[cd]pentalene (PIN)
read 2H-4,7,12-trioxa-1-thia-5,9b-[1,2]epicyclopentadicyclopenta[cd,h]azulene (PIN)
for 2H-5,9b-[2,3]furano-4,7-dioxa-1-thiacyclopenta[2′,3′:6,7]cyclohepta[cd]pentalene
read 2H-5,9b-[2,3]furano-4,7-dioxa-1-thiadicyclopenta[cd,h]azulene
Page 584, P-52.2.5.1 (1). [corrected 1 November 2023]
For (1) cyclophanes are cyclic phane structures containing one or more rings or ring systems, at least one ring or ring system of which must be a mancude system attached to adjacent atoms or chains at nonadjacent ring positions;
read
(1) cyclophanes are cyclic phane structures with at least six nodes including two or more rings or ring systems not ortho or ortho and peri-fused to the cyclophane ring, and with at least one ring or ring system of which must be a mancude system;
Page 584, P-52.2.5.2.1, example 2. [corrected 1 November 2023]
For 1(1,3)-benzenacyclopeptadecaphane (PIN; a phane name)
read 1(1,3)-benzenacyclopeptadecaphane (a phane name)
for bicyclo[16.3.1]docosa-1(22),18,20-triene (a von Baeyer name)
read bicyclo[16.3.1]docosa-1(22),18,20-triene (PIN; a von Baeyer name)
add Explanation: Only one ring system, see P-52.2.5.1 (1)
Page 586, P-52.2.5.2.1, example 1 on this page, right version. [corrected 29 May 2019]
For bicyclo[12.4.0]octadeca-(14),15,17-triene
read bicyclo[12.4.0]octadeca-1(14),15,17-triene
Page 586, P-52.2.5.2.1, example 2 on this page, name for (II).
For 5,7,14,16-tetrahydro-1,17:8,10-diethanodibenzo[c,j][1,8]dithiacyclotetradecine
read 5,7,14,16-tetrahydro-1,17:8,10-diethenodibenzo[c,j][1,8]dithiacyclotetradecine
Page 586, P-52.2.5.2.1, structure 3 on this page.
For (I) 3,6-dithia-1(1,7),5(7,1)-dinaphthalenacyclooctaphane (PIN;
read (I) 3,7-dithia-1(1,7),5(7,1)-dinaphthalenacyclooctaphane (PIN;
for (II) 5,7,14,16-tetrahydro-1,17:8,10-diethanodibenzo[c,j][1,8]dithiacyclotetradecine
read (II) 5,7,14,16-tetrahydro-1,17:8,10-diethenodibenzo[c,j][1,8]dithiacyclotetradecine
Page 587, P-52.2.5.3, example 1, second compound. [modified 20 February 2019]
For 3,5-bis([1,1′-biphenyl]-3-yl)pyridine (PIN, substitutive name)
read 3,5-di([1,1′-biphenyl]-3-yl)pyridine (PIN, substitutive name)
Page 587, P-52.2.5.3, example 1.
For 3,5-bis([11,21 :23,31-terphenyl]-13-yl)pyridine (a substitutive name, see P- 28.3.1)
read 3,5-di([11,21:23,31-terphenyl]-13-yl)pyridine (a substitutive name, see P-28.3.1) [delete space in name]
for 3,5-bis([1,1′ :3′,1′′-terphenyl]-3-yl)pyridine (a substitutive name)
read 3,5-di([1,1′:3′,1′′-terphenyl]-3-yl)pyridine (a substitutive name) [delete space in name]
Page 587, P-52.2.5.3, example 2. [modified 20 February 2019]
for (not diphenyl ether)
read diphenyl ether (a functional class name)
Page 588, P-52.2.5.3, example 3. [corrected 7 November 2018]
For 3,3′-[furan-3,4-diylbis(sulfanediylethane-2,1-diylsulfanediyl]difuran
read 3,3′-[furan-3,4-diylbis(sulfanediylethane-2,1-diylsulfanediyl)]difuran
Page 589, P-52.2.6.1, line 9 on this page. [corrected 29 January 2020]
For ...or a C70-D5h(6))[5,6]fullerene derived...
read ...or a (C70-D5h(6))[5,6]fullerene derived...p>
Page 589, P-52.2.6.1 (1) lines 3/4. [corrected 29 January 2020]
For ...and C70-D5h(6))[5,6]fullerene.
read ...and (C70-D5h(6))[5,6]fullerene.
Page 589, P-52.2.6.1 (2) line 4. [corrected 29 January 2020]
For ...and C70-D5h(6))[5,6]fullerene.
read ...and (C70-D5h(6))[5,6]fullerene.
Page 589, P-52.2.6.2, Example 1, structure (II). [corrected 19 September 2018]
For ...33,34,37,38-tricontanor(C60-Ih)[5,6]fullerene (II)
read ...33,34,37,38-triacontanor(C60-Ih)[5,6]fullerene (II)
Page 590, P-52.2.6.2, Example 2. [corrected 29 January 2020]
For [not bis(benzo[1,8]-as-indaceno[3,4,5,6-fghij:3′4′5′6′-lmnoa])cyclopenta[cd]fluoranthene (II)]
read [not bis(benzo[1,8]-as-indaceno[3,4,5,6-fghij:3′,4′,5′,6′-lmnoa])cyclopenta[cd]fluoranthene (II)]
Page 591, P-52.2.6.2, Example 4. [modified 29 January 2020]
For [not 16H-1,13,18-(epiethFor [not 16H-1,13,18-(epiethene[1,2]diyl[1]ylidene)-2,11,12-epiprop[1]ene[1,3]diyl[3]ylidene)acephenanthryleno[4,3-bc]tricyclopenta[n,pqr,tuv]picene (II)]
read [not 8,7,2-(epiethane[1,2]diyl[1]ylidene)-1,9,18-(epiprop[1]ene[1,1]diyl[3]ylidene)acephenanthryleno[4,3-bc]tricyclopenta[n,pqr,tuv]picene (II)]
(II)]
replace the structures with:
Page 593, P-52.2.6.4, Example 2. [corrected 29 January 2020]
For 2b2,8a2:5,8-dimethenecycclohexadeca[1,2,3,4,5,6-cdefg:7,8,9,10,11,12-c′d′e′f′g′]di-as-indacene (I) (PIN)
read 3,2,13,12-(epihexa[1,3,5]trien[1,3,4,6]tetrayl)-6,9-methenocycloundeca[1,11,10-cd:6,7,8-c′d′]diindene (I) (PIN)
Replace the structures with:
Page 594, P-52.2.6.4, Example 3. [corrected 29 January 2020]
Reverse the structures for (I) and (II).
for 2,15:3,14-dimethenobenzo[h]cyclododeca[1,2-a:6,7-a′]difluorene (I)(PIN)
read 2,15:3,14-dimethenoindeno[5'',4'':6',7']cyclododeca[1',2':4,5]indeno[1,2-b]anthracene (PIN)
replace the structure for (I) [previously (II)] with:
Page 595, P-52.2.7.1, example 2 on page. [modified 3 June 2020]
For 2,2′-bi(bicyclo[2.2.2]octan)-2-yl
read 2,2′-bi(bicyclo[2.2.2]octanyl)
Page 595, P-52.2.7.2, example 1, left hand structure.
Replace structure with:
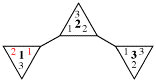
Page 596, P-52.2.7.3, example on this page. [corrected 19 September 2018]
For 1,8(2),2,3,4,5,6,7(2,5)-heptathiophenaoctaphane (PIN)
read 1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
Page 596, P-52.2.8, last example.
For (a) di(tridecyl)benzene
read 1,2-di(tridecyl)benzene
for [not 1,2-phenylenedi(tridecane);
read [not 1,1′-(1,2-phenylene)di(tridecane);
Page 597, P-54.2, example 2. [modified 4 September 2024]
Replace the structure with:

for cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
read (1Z,3E,5Z,7E,9Z)-cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
for 1,2-didehydro[12]annulene
read 1,2-didehydro[12]annulene (numbering shown)
Page 598, P-54.3, example 2. [modified 6 December 2023]
Replace the structure with:
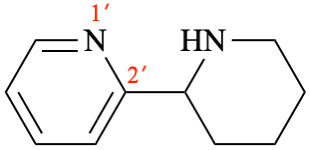
read (1Z,3E,5Z,7E,9Z)-cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
for 1,2-didehydro[12]annulene
read 1,2-didehydro[12]annulene (numbering shown)
Page 598, P-54.3, example 3. [modified 29 January 2020]
For 4-[4-(4′-phenyl-[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexyl
read 14-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexyl
Page 600, P-54.4.2, example 6 on this page.
For 1,4-thiazepinane (PIN)
read 1,4-thiazepane (PIN)
Page 600, P-54.4.2, example 7 on this page. [corrected 27 May 2020]
Delete pentazolane
Page 601, P-54.4.3.1 box, line 3. [corrected 8.11.2023]
For ...see also P-15.1.3.2, P-31.2, P-58.2), ...
read ...see also P-15.1.5.2, P-31.2, P-58.2), ...
Page 601, P-54.4.3.1, last structure on this page. [corrected 14 November 2018]
For 1,2-dihydro2,2′-binaphthyl
read 1,2-dihydro-2,2′-binaphthyl
Page 603, P-55, paragraph 5 lines 2/3. (corrected 4 December 2019)
For ...except anisole and tert-butoxy, formic acid, and the formyl group are substitutable with limitations.
read ...except anisole and tert-butoxy, and formic acid and formyl group are substitutable with limitations.
Page 604, P-56.2, lines 3/4. [modified 20 February 2019]
For ...‘SO-thioperoxol’, SeO-selenoperoxol’, ‘SS-dithioperoxol’, TeS-tellurothioperoxol’, ‘SeSe-diselenoperoxol’, ‘SeTe-selenotelluroperoxol’, and ‘TeSe-selenotelluroperoxol’ (see P-63.4.2.1).
read ...‘SO-thioperoxol’, ‘SeO-selenoperoxol’, ‘dithioperoxol’, ‘TeS-tellurothioperoxol’, ‘diselenoperoxol’, ‘SeTe-selenotelluroperoxol’, and ‘TeSe-telluroselenoperoxol’ (see P-63.4.2.1).
Page 604, P-56.2, box line 2.
For ...suffix ‘sulfenic aced and it chalcogen...
read ...suffix ‘sulfenic acid’ and its chalcogen...
Page 605, P-56.4, line 3 on this page.
For ...rather thanmethanediyl. CAS...
read ...rather than methanediyl. CAS... [insert space between words]
Page 605, P-56.4, last example.
For (carbonyldiimino, a name...
read (not carbonyldiimino, a name...
Page 606, P-57.1.1.1, example 4. [corrected 31 October 2018]
For 1-propanylidene
read propan-1-ylidene
Page 607, P-57.1.3, line 3.
For ... isopropylidene for (CH3)C=, ...
read ... isopropylidene for (CH3)2C=, ...
Page 607, P-57.1.4, line 2.
For (2-methylbutyl)
read (2-methylpropyl)
Page 608, P-57.1.5.3, example 2. [corrected 19 September 2018]
For anthacen-2-yl (preferred prefix)
read anthracen-2-yl (preferred prefix)
Page 608, P-57.1.5.3, example 4.
For 7-isoquinolyl (also 1-, 3-, 5-, 6-, 7-, and 8- isomers)
read 7-isoquinolyl (also 1-, 3-, 4-, 5-, 6-, and 8-isomers)
for isoquinol-7-yl (also 1-, 3-, 5-, 6-, 7-, and 8- isomers, preferred prefixes)
read isoquinol-7-yl (also 1-, 3-, 4-, 5-, 6-, and 8-isomers, preferred prefixes)
Page 609, P-57.1.5.3, example 1 on this page.
For naphthalen-2-yl (also 2-isomer;
read naphthalen-2-yl (also 1-isomer;
Page 613, P-57.4, example 1 Explanation lines 3 and 4.
For ‘Benzyl’ is chosen; it is larger than ‘methyl’ leading to the prefix ‘phenylmethoxy’.
read ‘Benzyl’ is chosen; it is larger than ‘methyl’ leading to the prefix ‘benzyloxy’.
Page 613, P-57.4, example 3. [modified 3 June 2020]
For 4-[4-(benzyloxy)phenyl]methoxy (prefered prefix)
read [4-(benzyloxy)phenyl]methoxy (preferred prefix)
Page 614, P-57.4, example 1 on this page. [corrected 3 June 2020]
For [1,1′-biphenyl]-4-yloxy (preferred prefix)
read ([1,1′-biphenyl]-4-yl)oxy (preferred prefix)
Page 614, P-57.4, example 1 on this page, explanation, lines 4/5. [corrected 3 June 2020]
For ...which results in the prefix ‘[1,1′-biphenyl]-4-yloxy’.
read ...which results in the prefix ‘([1,1′-biphenyl]-4-yl)oxy’.
Page 614, P-57.4, example 2 on this page. [corrected 7 November 2018]
For [not (dimethylamino)carbonyl]hydrazinylidene]
read [not [(dimethylamino)carbonyl]hydrazinylidene]
Page 615, P-58.1, example 1, Explanation line 1. [corrected 21 August 2019]
For ...name 2-(3-cyanophenoxy])4-isopropylbenzonitrile would...
read ...name 2-(3-cyanophenoxy)-4-isopropylbenzonitrile would...
Page 615, P-58.1, example 2.
For 4-chloro-2-(1,3-oxazol-5-ylmethyl)-1,3-oxazole (PIN)
read 4-chloro-2-[(1,3-oxazol-5-yl)methyl]-1,3-oxazole (PIN)
Page 616, P-58.2.1.2, example 4. [corrected 26 May 2021]
For 1H,2H-3a,7a-methano[2]benzofuran (PIN)
read 1H,2H-3a,7a-methano-2-benzofuran (PIN)
Page 617, P-58.2.1.2, example 1 on this page. [corrected 25 August 2016]
For (not 2,3-dihydro-4H-1-benzoyran;...
read (not 2,3-dihydro-4H-1-benzopyran;...
Page 619, P-58.2.2.3, example 3. [corrected 19 September 2018]
For napthalene-4a,8a-diyl (preferred prefix)
read naphthalene-4a,8a-diyl (preferred prefix)
Page 620, P-58.2.3.1.1, example 1. [corrected 21 November 2018]
For tetrahydro-4H-pyran-4-one (PIN)
read tetrahydro-4H-pyran-4-one
add oxan-4-one (PIN)
Page 620, P-58.2.3.1.1, example 2. [corrected 20 February 2019]
For 2-(1,3,4,5-tetrahydro-2H-2-benzazepin-2-yl)ethanol (PIN)
read 2-(1,3,4,5-tetrahydro-2H-2-benzazepin-2-yl)ethan-1-ol (PIN)
Page 621, P-58.2.3.1.1, example 1 on this page. [corrected 19 September 2018]
For 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c]cycloheptene-6,6-dicarboxylic acid (PIN)
read 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c][7]annulene-6,6-dicarboxylic acid (PIN)
add [not 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c]cycloheptene-6,6-dicarboxylic acid]
Page 622, P-58.2.3.1.2, example 1. [modified 27 February 2019]
For dihydro-1H,3H,4H-3a, 6a-methanocyclopenta[c]furan-1,3-dione (PIN)
read 5,6-dihydro-1H,3H,4H-3a,6a-methanocyclopenta[c]furan-1,3-dione (PIN) [delete space in name]
for (not 1H,3H,6H-3a,6a-methanocyclopenta[c]furan-1,3-dione; ...
read (not 4,5-dihydro-1H,3H,6H-3a,6a-methanocyclopenta[c]furan-1,3-dione; ...
Page 622, P-58.2.3.1.2, example 2. [corrected 29 January 2020]
For (not 2H,8H-benzo[1,2-b:5,4-b′]dipyran-4(3H)-one;
read (not 6,7-dihydro-2H,8H-benzo[1,2-b:5,4-b′]dipyran-4(3H)-one;
for 2H,6H’ is lower than ‘2H,8H’)
read ‘2H,6H’ is lower than ‘2H,8H’)
Page 624, P-58.2.3.1.4, example 2. [corrected 14 November 2018]
For (not 4,4a-dihydro 2H,4H-benzo[1,2-b:4,3-c′]dipyran-5,6(4aH,6aH)-dione)
read (not 4,4a-dihydro-2H,4H-benzo[1,2-b:4,3-c′]dipyran-5,6(4aH,6aH)-dione)
Page 625, P-58.2.3.1.4, example 1 on this page, Explanation, lines 2/3. [corrected 7 November 2018]
For ... positions; (6H,8H)-pyrido[3,2,1-ij]quinoline is not a permissible structure), so ...
read ... positions; (6H,8H-pyrido[3,2,1-ij]quinoline is not a permissible structure), so ...
Page 625, P-58.2.4, example 1.
For 1,3-dihydro-1,3-dioxo-2H-isoindole-2,5-diyl (preferred prefix)
read 1,3-dioxo-1,3-dihydro-2H-isoindole-2,5-diyl (preferred prefix)
Page 627, P-58.3.2, example 2. [modified 3 June 2020]
For N-triazan-1-ylbenzamide (PIN)
read N-(triazan-1-yl)benzamide (PIN)
for (not phenyl(tetraazan-1-yl)methanone; nor 1-benzoyltetraazane)
read [not phenyl(tetraazan-1-yl)methanone; nor 1-benzoyltetraazane]
Page 627, P-58.3.2, example 3. [modified 3 June 2020]
For tetrazane-1-carboxylic acid (PIN)
read tetraazane-1-carboxylic acid (PIN)
for (not triazan-1-ylcarbamic acid; the carboxylic acid is senior to the carbonic acid derivative)
read [not (triazan-1-yl)carbamic acid; the carboxylic acid is senior to the carbonic acid derivative]
Page 627, P-58.3.2, example 7.
For [not 1-(oxomethylidene)carbonyltetrazane]
read [not 1-(oxomethylidene)tetraazane]
Page 627, P-58.3.2, example 8.
For N,N′-hydrazine-1,2-diyldibenzamide (PIN)
read N,N′-(hydrazine-1,2-diyl)dibenzamide (PIN)
Page 627, P-58.3.2, example 9. [corrected 24 March 2021]
For N-isocyanatonitramide
read isocyanatonitramide
Page 628, P-58.3.2, example 1 on this page. [corrected 19 September 2018]
For [not trisulfanyl benzenecarbothiaote;
read [not trisulfanyl benzenecarbothioate;
Page 630, P-59.1.9, Table 5.1, column 3, line 12. [corrected 7 November 2018]
For –S(O2)-R2,6
read –S(O)2-R2,6
Page 630, Table 5.1, Footnote 5. [corrected 20 February 2019]
For ... as thiocyanate and selenocyanate.
read ... as isothiocyanate and isoselenocyanate.
Page 631, P-59.1.9, paragraph 4, line 9 on this page. [corrected 29 January 2020]
For ...propan-2-ol (mot propane-2-ol).
read ...propan-2-ol (not propane-2-ol).
Page 633, P-59.2.1.2, Analysis, line 5. [corrected 26 September 2018]
For CH3-CH2-CH=CH2CH2-COOH
read CH3-CH2-CH=CH-CH2-COOH
Page 634, P-59.2.1.3, Example 1 Functionalized parent hydride. [corrected 29 January 2020]
For ethanediol
read ethane-1,2-diol
Page 635, P-59.2.1.3, example 3, line 4.
For 1-propanol
read propan-1-ol
Page 637, P-59.2.1.6, first structure on this page.
Replace structure with:
Page 639, P-59.2.1.8, third structure on this page.
Replace structure with:
-CH2-CH2-CH2-CH2-CH2-CH3
Page 644, p-59.2.3.3, Example 3.
This example should be deleted. The PIN for this example is oxan-3-one. It should be replaced with:
Analysis: Principal characteristic group: =O dione Parent hydride: 1H-indene Functionalised parent hydride: 1H-indene-1,4(2H)-dione Saturation prefix: dihydro
Together with other rules, this analysis leads to the preferred IUPAC name: 3,3a-dihydro-1H-indene-1,4(2H)-dione
Page 645, P-59.2.3.3, example 4 on this page, last line.[corrected 20 February 2019]
For 3a,9b-dihydro-3H-cyclopenta[a]naphthalene-3,5(2H)-dione
read 3a,9b-dihydro-1H-cyclopenta[a]naphthalene-3,5(2H,4H)-dione
Page 654, P-61.2.3, example 3 on this page.
Add allylcyclohexane
Page 654, P-61.2.3, example 6 on this page.
For 1,3-di(propan-2-yl)cyclohexane (PIN)
read 1,4-di(propan-2-yl)cyclohexane (PIN)
Page 654, P-61.2.3, example 8 on this page.
For {not [1-methyl-3-(4-methylcyclohex-3-en-1-yl) prop-2-en-1-y]benzene;
read {not [1-methyl-3-(4-methylcyclohex-3-en-1-yl)prop-2-en-1-yl]benzene; [delete space from name]
Page 655, P-61.2.3, example 1 on this page. [modified 27 February 2019]
For 1,1′,1′′-(benzene-1,2,4-triyltripropane-3,1-diyl)tris(4-methylbenzene) (PIN)
read 1,1′,1′′-[benzene-1,2,4-triyltri(propane-3,1-diyl)]tris(4-methylbenzene) (PIN)
Delete 1,2,4-tris(3-p-tolylpropyl)benzene
Page 655, P-61.2.3, last example on this page. [corrected 27 February 2019]
For 2,3,7,8-tetra(naphthalen-2-yl)anthracene
read 2,3,6,7-tetra(naphthalen-2-yl)anthracene
replace the structure with:
Page 656, P-61.2.4, example 2.
For 2,6-bis(benzo[a]anthracen-1-yl)pyridine (PIN)
read 2,6-bis(benzo[a]anthracen-1-yl)pyridine
add 2,6-di(tetraphen-1-yl)pyridine (PIN)
Page 657, P-61.3.1, example 5.
Add 1,4-bis(1-chloro-1-methylethyl)benzene
Page 658, P-61.3.1, example 6 on this page.
For 1-(5-bromopent-2-en-2-yl)cyclopropane
read (5-bromopent-2-en-2-yl)cyclopropane
for 1-(4-bromo-1-methylbut-1-en-1-yl)cyclopropane
read (4-bromo-1-methylbut-1-en-1-yl)cyclopropane
Page 659, P-61.3.1, examples 2 and 3, I and II. [modified 5 June 2019].
For C60Ih
read C60-Ih
replace the structure of I with:
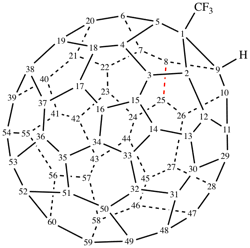
Replace the structure of II with:
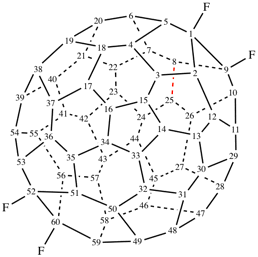
Page 660, P-61.3.2.1, example 1. [corrected 27 February 2019]
For chloro(dimethyl)borane (PIN)
read chlorodi(methyl)borane (PIN)
Page 661, P-61.3.2.2, example 2. [corrected 7 November 2018]
For chloro(methyl)phosphane]
read chloro(methyl)phosphane
Page 661, P-61.3.2.2, example 1. [corrected 21 April 2021]
For N-methylhypochlorous amide (PIN; see P-67.1.2.6)
read methylhypochlorous amide (PIN; see P-68.5.3)
Page 662, P-61.4, example 3.
Replace the structure with:
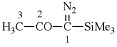
Page 664, P-61.6, example 2.
For N-(2-methylpropyl)oxoarsanamine (PIN)s
read N-(2-methylpropyl)-1-oxoarsanamine
Page 665, P-61.8, example 3.
For 4-isocyanatobenzenesulfonyl chloride (PIN)
read 4-isocyanatobenzene-1-sulfonyl chloride (PIN)
Page 665, P-61.9, example 1. [corrected 26 September 2018]
Replace the structure with:
C6H5-NC
Page 666, P-61.10, example 3. [corrected 24 July 2019]
For λ2-methylidenehydroxylamine (PIN)
read λ2-methylidenehydroxylamine
add N-hydroxy-λ2-methanimine (PIN)
Page 670, P-62.2.1.2, example 4. [corrected 19 September 2018]
For (quinolin-4-yl)amine
read (quinolin-4-yl)azane
Page 670, P-62.2.1.2, example 6.
For 1-thiacyclotridecan-3-yl)azane
read (1-thiacyclotridecan-3-yl)azane
Page 671, P-62.2.1.3, example 2.
For trimethylsilamamine (PIN)
read 1,1,1-trimethylsilanamine (PIN)
Page 671, P-62.2.2.1, paragraph 4.
For ‘formed by method (2) are set off by parentheses’
read ‘formed by method (3) are set off by parentheses’
Page 671, P-62.2.2.1, example 2.
For N,N-diethylethan-1-amine
read N,N-diethylethanamine
for triethlazane
read triethylazane
Page 672, P-62.2.2.1, example 4 on this page. [modified 18 September 2019]
For (1) N-silylsilanamine
read (1) N-silylsilanamine (preselected name)
Page 673, P-62.2.2.2, example 4 on this page. [corrected 24 June 2020]
Delete (not 2,2′-dimethyl-N-(2,2-dimethylpropyl)di(prop-2-en-1-amine)
Page 673, P-62.2.2.2, example 7 on this page. [modified 26 January 2022]
For N-butylcyclopropan-1-amine (PIN)
read N-butylcyclopropanamine (PIN)
For N-cyclopropylbutan-1-amine
read (not N-cyclopropylbutan-1-amine)
Page 674, P-62.2.2.2, example on this page. [corrected 24 June 2020]
For (not 5,6,7,8-tetrahydrodi-2-naphthylamine)
read [not 5,6,7,8-tetrahydrodi(2-naphthyl)amine]
Page 675, P-62.2.3, example 2 on this page. (modified 4 March 2020)
For (not disilazan-2-yl acetic acid)
read [not (disilazan-2-yl)acetic acid]. [deleted space from name]
Page 675, P-62.2.4.1.1, example 2. [corrected 7 November 2018]
For [(4′-amino[1,1′-biphenyl]-4-yl)amino]
read (4′-amino[1,1′-biphenyl]-4-yl)amino
Page 676, P-62.2.4.1.2, example 1. [corrected 23 February 2022)
Replace structure by
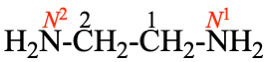
Page 677, P-62.2.4.1.3, example 2. [corrected 24 October 2018]
Replace the structure with:
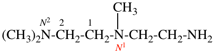
Page 678, P-62.2.4.1.3, example 1 on this page. [modified 27 February 2019]
For N1,N1′-(azanediyldiethane-2,1-diyl)di(ethane-1,2-diamine) [PIN;...
read N1,N1′-[azanediyldi(ethane-2,1-diyl)]di(ethane-1,2-diamine) [PIN;...
delete N1-(2-aminoethyl)-N2-{2-[(aminomethyl)amino]ethyl}ethane-1,2-diamine
(substitutive name; alphabetically inferior to the first substitutive name]
Page 678, P-62.2.4.1.3, example 3 on this page. [corrected 27 February 2019]
For N1,N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
read N1,N2-bis{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine >
Page 678, P-62.2.4.1.3, example 4 on this page.
For N1,N1′-(cyclohexane-1,4-diyldiethane-2,1-diyl)di(propane-1,3-diamine) [PIN...
read N1,N1′-[1,4-phenylenedi(ethane-2,1-diyl)]di(propane-1,3-diamine) [PIN...
Page 679, P-62.2.4.1.3, example on this page. [corrected 27 February 2019]
For N1-(4-aminophenyl)-N1,N1′-(azanediyldi-1,4-phenylene)di(benzene-1,4-diamine)
read N1-(4-aminophenyl)-N1,N1′-(azanediyldi-4,1-phenylene)di(benzene-1,4-diamine)
Add N4-[4-(4-aminoanilino)phenyl]-N1,N1-bis(4-aminophenyl)benzene-1,4-diamine
Page 679, P-62.2.4.1.3, example on this page [corrected 9 April 2025]
For N1-(4-aminophenyl)- N1,N1′-(azanediyldi-4,1-phenylene)di(benzene-1,4-diamine)
read N1-(4-aminophenyl)- N1,N1′-[azanediyldi(4,1-phenylene)]di(benzene-1,4-diamine)
Page 679, P-62.2.5.1, line 3.
For ...reserved to denot e only the...
read ...reserved to denote only the... [remove space in word]
Page 679, P-62.2.5.1, example 2.
Replace structure with:
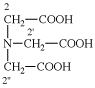
Page 679, P-62.2.5.1, example 1. [corrected 27 February 2019]
Add 4-(4-cyanoanilino)benzonitrile
Page 680, P-62.2.5.2 (1), example. [modified 5 June 2019]
For N,N-diethylmethanediamine (PIN)
read N,N′-diethylmethanediamine
for N,N′-methylenedi(ethan-1-amine)
read N,N′-methylenediethanamine (PIN)
replace structure with:
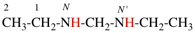 Page 680, P-62.2.5.2 (2) (ii), example 2.
Page 680, P-62.2.5.2 (2) (ii), example 2.
For N1,N1′--methylenedi(benzene-1,4-diamine)
read N1,N1′-methylenedi(benzene-1,4-diamine)
Page 681, P-62.2.5.3, example.
For 2,2′-methylenebis[N12-methyl-1,4(1,4)dibenzenacyclohexaphane-12,13-diamine
read 2,2′-methylenebis[N12-methyl-1,4(1,4)dibenzenacyclohexaphane-12,13-diamine]
Page 682, P-62.2.6.2, example 2 on this page.
For (5,6,7,8-tetrahydronaphthalen-2-y l)azane
read (5,6,7,8-tetrahydronaphthalen-2-yl)azane [delete space in name]
for (5,6,7,8-tetrahydronaphthalen-2-y l)amine
read (5,6,7,8-tetrahydronaphthalen-2-yl)amine [delete space in name]
Page 682, P-62.2.6.2, example 4 on this page. [modified 27 February 2019]
For 2,4a-dihydronaphthalene-2,4a-diamine
read 2,4a-dihydronaphthalene-2,4a-bis(azane)
for 2,4a-dihydronaphthalen-4a-amine
read 2,4a-dihydronaphthalene-2,4a-diamine
add naphthalene-2,4a(2H)-bis(azane)
Page 683, P-62.3.1.1, example 5 on this page. [modified 27 February 2019]
For N,N′-dimethylnaphthalene-1,4-diimine (PIN; see also P-58.2.2.3)
read N,N′-dimethylnaphthalene-1,4-diimine (see also P-58.2.2.3)
Add N1,N4-dimethylnaphthalene-1,4-diimine (PIN; see also P-16.9.2)
for [not N,N′-naphthalene-1,4-lidenebis(methanamine)]
read [not N,N′-(naphthalene-1,4-diylidene)bis(methanamine)]
for [not N,N′naphthalene-1,4-diylidenebis(methylamine)]
read [not N,N′-(naphthalene-1,4-diylidene)bis(methylamine)]
for dimethyl(napththalene-1,4-diylidene)bis(amine)]
read dimethyl(naphthalene-1,4-diylidene)bis(amine)]
replace the structure with:
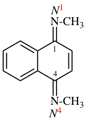
Page 684, P-62.3.1.3, example 1.
For methylphosphanimine
read 1-methylphosphanimine
Page 685, P-62.4, box, lines 3-5. [corrected 24 July 2019]
For ...(see P-67.1.2.6) . Compounds such as R-NH-OH are named as N-derivatives of hydroxylamine, NH2-OH (see P-68.3.1.1.1). Names based on N-substitution of amines are not recommended in these cases.
read ...(see P-67.1.2.6). Compounds such as R-NH-OH are named as N-derivatives of the senior amine (see P-68.3.1.1.1).
Page 685, P-62.4, example 1.
For N-methoxyethan-1-amine (PIN, see P-68.1.1.3)
read N-methoxyethanamine (PIN, see P-68.1.1.1.3)
Page 685, P-62.4, example 2.
For N-ethylhydroxylamine (PIN)
read N-ethylhydroxylamine
add N-hydroxyethanamine (PIN)
Page 685, P-62.4, example 3. [corrected 7 April 2021]
For N-ethylhypochlorous amide (PIN)
read ethylhypochlorous amide (PIN)
Page 685, P-62.4, example 3. [corrected 5 June 2019]
For N-chloroethan-1-amine
read N-chloroethanamine
Page 685, P-62.4, example 4. [modified 21 April 2021]
For N-methylnitrous amide (PIN)
read methylnitrous amide (PIN)
for (not N-nitrosomethanamine)
read N-nitrosomethanamine
Page 685, P-62.4, example 5. [modified 21 April 2021]
For N-methyl-N-nitronitramide (PIN)
read methyl(nitro)nitamide (PIN)
for (not N,N-dinitromethanamine)
read N,N-dinitromethanamine
Page 685, P-62.4, example 6. [corrected 21 April 2021]
For N-methylbromous amide (PIN)
read methylbromous amide (PIN)
for (not N-bromosylmethanamine)
read N-bromosylmethanamine
Page 697, P-63.1.5, line 9. [corrected 8.11.2023]
For ...are described in P-63.2.5.1. multiplicative names ...
read ...are described in P-63.2.5. multiplicative names ...
Page 687, P-62.5, example 1 on this page. [modified 27 February 2019]
For 3-carbamoyl-5-(2-aminoethyl)benzoic acid N5-oxide
read 3-(2-aminoethyl)-5-carbamoylbenzoic acid N3-oxide
For {not {[2-(3-carbamoyl-5-carboxyphenyl)ethyl]azaniumyl}oxidanide (see also P-71.2.1.2}
read (3) {[2-(3-carbamoyl-5-carboxyphenyl)ethyl]azaniumyl}oxidanide (see also P-71.2.1.2)
Page 687, P-62.5, example 3 on this page. [modified 7 November 2018]
For (2) 2-{3-[(dimethyloxo-λ5- azanyl)methyl]phenyl}-N,N-dimethyl-ethan-1-amine N-oxide
read (2)
2-(3-{[dimethyl(oxo)-λ5-azanyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide (PIN) [Deleted space and a hyphen from the name]
for 2-{3-[(dimethylamino)methyl]phenyl}-N,N-dimethylethane-1-amine N1,N3-dioxide
read 2-{3-[(dimethylamino)methyl]phenyl}-N,N-dimethylethan-1-amine N1,N3-dioxide
for (3) 2-(3-{[dimethyl(oxido)azaniumyl]methyl}phenyl)-N,N-dimethyl-ethan-1-amine N-oxide (PIN; see also P-74.2.1.2)
read (3) 2-(3-{[dimethyl(oxido)azaniumyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide (see also P-74.2.1.2)
Replace the structure with:
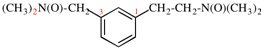
Page 687, P-62.5, last example.
For N,N-diethylethan-1-amine N-sulfide (PIN)
read N,N-diethylethanamine N-sulfide (PIN)
Page 688, P-62.6.1, example 3. [modified 11 November 2020]
For (2) ethyldimethylazanium iodide
read (2) ethyldi(methyl)azanium iodide
delete (3) ethyldimethylammonium iodide
Page 688, P-62.6.1, example 5.
For N-methylbenzenaminium bromide (PIN)
read N-methylbenzenaminium bromide
add N-methylanilinium bromide (PIN)
Page 688, P-62.6.1, example 7.
For N-methylethan-1-iminium chloride (PIN)
read N-methylethaniminium chloride (PIN)
Page 688, P-62.6.1, last example.
For N-phenylethan-1-iminium bromide (PIN)
read N-phenylethaniminium bromide (PIN)
Page 689, P-62.6.2, example 2 on this page. [corrected 26 September 2018]
Replace the structure with:
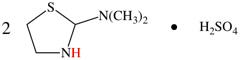
Page 689, P-63.0, lines 5/6. [corrected 27 February 2019]
For ...For instance, H2Si-OH is classified...
read ...For instance, H3Si-OH is classified...
Page 690, P-63.1, line 12.
Delete P-63.1.6 Polyfunctional hydroxy compounds
Page 692, P-63.1.2, example 4 on this page. [corrected 26 September 2018]
Replace the structure with:
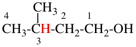
Page 694, P-63.1.2, example 1 on this page. [modified 27 February 2019]
For (C60-Ih)[5,6]fulleren-1(9H)-ol (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ol (PIN)
For 1,9-dihydro(C60-Ih)[5,6] fulleren-1-ol
read 1,9-dihydro(C60-Ih)[5,6] fulleren-1-ol
replace the structure with:
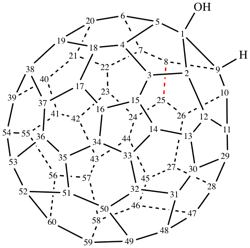
Page 694, P-63.1.2, example 3 on this page.
For (1) 2-fluoro-6-[(2-{[(3-fluoro-2-hydroxyphenyl)methylidene]amino}ethyl)imino]methyl}phenol
read (1) 2-fluoro-6-{[(2-{[(3-fluoro-2-hydroxyphenyl)methylidene]amino}ethyl)imino]methyl}phenol
Page 696, P-63.1.3, example 1. [corrected 27 February 2019]
For hydroxy(trimethyl)silane
read hydroxytri(methyl)silane
Page 697, P-63.1.5, line 9. [corrected 8.11.2023]
For ...are described in P-63.2.5.1. multiplicative names ...
read ...are described in P-63.2.5. multiplicative names ...
Page 700, P-63.2.2.1.1, example 2. [corrected 5 June 2019]
For butan-2-yloxy
read (butan-2-yl)oxy
Page 700, P-63.2.2.1.1, example 3. [corrected 5 June 2019]
For pyridin-2-yloxy
read (pyridin-2-yl)oxy
Page 700, P-63.2.2.1.1, example 4. [corrected 5 June 2019]
For piperidin-2-yloxy
read (piperidin-2-yl)oxy
Page 700, P-63.2.2.1.1, example 5. [corrected 5 June 2019]
For 2-(butan-2-yloxy)ethyl
read 2-[(butan-2-yl)oxy]ethyl
Page 701, P-63.2.2.2, last example on this page. [corrected 5 June 2019]
For propan-2-yloxy
read (propan-2-yl)oxy
Page 702, P-63.2.2.2, example 1 on this page. [corrected 5 June 2019]
For butan-2-yloxy
read (butan-2-yl)oxy
Page 702, P-63.2.3, example 3. [corrected 27 February 2019]
For (not 2-methoxyanisole; see P-34.1.1.2 and P-15.1.8.2 for substitution rules for anisole)
read 2-methoxyanisole (see P-34.1.1.4 and P-15.1.8.2 for substitution rules for anisole)
Page 704, P-63.2.4.2, example 3.
For 2-(pyridin-3-yloxy)pyrazine (PIN)
read 2-[(pyridin-3-yl)oxy]pyrazine (PIN)
Page 705, P-63.2.4.2, example 2 on this page.
For (3) 2,4′-dichloro-1,1′-oxydibenzene
read (3) 2,4′-dichloro-1,1′-oxydibenzene (numbering shown)
Page 706, P-63.2.5.1, example 5.
For (1) 1-[(penta-1,4-dien-3-yl)sulfanyl)]cyclobutane (PIN)
read (1) [(penta-1,4-dien-3-yl)sulfanyl]cyclobutane (PIN)
for 1-[(penta-1,4-dien-3-yl)thio)]cyclobutane
read [(penta-1,4-dien-3-yl)thio]cyclobutane
Page 706, P-63.2.5.1, example 6.
For (1) 2-{[(methylsulfanyl)methyl]sulfanylthio}-1-[({[(methylsulfanyl)methyl]sulfanyl}methyl)sulfanyl]propane
read (1) 2-{[(methylsulfanyl)methyl]sulfanyl}-1-[({[(methylsulfanyl)methyl]sulfanyl}methyl)sulfanyl]propane
Page 707, P-63.2.5.1, example 1 on this page.
For (1) 1-(propan-2-ylselanyl)-2-(propylselanyl)propane (PIN)
read (1) 1-[(propan-2-yl)selanyl]-2-(propylselanyl)propane (PIN)
for 1-(propan-2-ylseleno)-2-(propylseleno)propane
read 1-[(propan-2-yl)seleno]-2-(propylseleno)propane
Page 707, P-63.2.5.1, example 2 on this page. [corrected 5 July 2018]
For 1-phenoxy-3-[[3-(phenylselanyl)phenyll]sulfanyl]benzene (substitutive name)
read 1-phenoxy-3-{[3-(phenylselanyl)phenyl]sulfanyl}benzene (substitutive name)
for 3-[3-(phenoxyphenyl)sulfanyl]-1-(phenylselanyl)benzene (substitutive name)
read not 1-[(3-phenoxyphenyl)sulfanyl]-3-(phenylselanyl)benzene (substitutive name)
for (the first substitutive name would be preferred because phenoxy-phenylsulfanyl is lower alphabetically than phenoxyphenyl-sulfanyl)
read (the first substitutive name is correct because phenoxy-phenylselanyl is lower alphabetically than phenoxyphenyl-sulfanyl)
Page 708, P-63.3.1, example 4. [corrected 24 June 2020]
For (1) 1-(propan-2-yldiselanyl)propane (PIN)
read (1) 1-[(propan-2-yl)diselanyl]propane (PIN)
Page 708, P-63.3.1, last example on this page. [corrected 19 September 2018]
For 1-(methyldiseleno)-2-(methyldithio)disilane
read 1-(methyldiseleno)-2-(methylditelluro)disilane
Page 709, P-63.3.1, example 1 on this page. [corrected 7 November 2018]
For [4-(4-carboxyphenyl)peroxy]benzoic acid
read 4-[(4-carboxyphenyl)peroxy]benzoic acid
Page 709, P-63.3.1, example 3 on this page. [corrected 27 February 2019]
Delete
(1) 1-(2-{[2-(methylsulfanyl)ethyl]sulfanyl}ethyl)-2-[(methylsulfanyl)methyl]disulfane
Page 709, P-63.3.1, example 4 on this page. [corrected 27 February 2019]
Delete (1) bis[3-(phenylsulfanyl)phenyl]disulfane
Page 710, P-63.3.2, example 3 on this page. [corrected 7 November 2018]
For {not phenyltellanyl)selanyl]benzene; ...
read {not [(phenyltellanyl)selanyl]benzene; ...
Page 710, P-63.3.2, example 4 on this page. [corrected 27 February 2019]
Delete (1) 1-[2-({[2-(methylselanyl)ethyl]sulfanyl}ethyl)]-2-[(methylsulfanyl)methyl]disulfane
for [not (methylsufanyl)methyl 2-{[2-(methylsulfanyl)ethyl]sulfanyl}ethanesufenothioate
read [not (methylsufanyl)methyl 2-{[2-(methylselanyl)ethyl]sulfanyl}ethanesufenothioate]
Page 710, P-63.3.2, example 5. [modified 24 June 2020]
Delete (1) [3-(phenyltelluranyl)phenyl][3-(phenylsulfanyl)phenyl]disulfane
for (not 3-(phenylsulfanyl)phenyl 3-(phenyltelluranyl)benzenesulfenothioate)
read [not 3-(phenylsulfanyl)phenyl 3-(phenyltellanyl)benzenesulfenothioate]
Page 711, P-63.4.1, example 3.
For 3-(dimethylamino)-2-methylbutan-2-yl hydroperoxide
read 4-(dimethylamino)-2-methylbutan-2-yl hydroperoxide
Page 712, P-63.4.2.1, Table 6.1.
For O-thioperoxol
read -SO-thioperoxol
for -TeSe-elenotelluroperoxol
read -TeSe-selenotelluroperoxol
Page 713, P-63.4.2.2, example 1.
For 2-hydroperoxyethanol (PIN)
read 2-hydroperoxyethan-1-ol (PIN)
Page 716, P-63.6, example 1 on page.
For sulfinyldibenzene (PIN)
read 1,1′-sulfinyldibenzene (PIN)
Page 716, P-63.6, example 2 on page.
For selenonyldibenzene (PIN)
read 1,1′-selenonyldibenzene (PIN)
Page 716, P-63.6, last example.
For (ethylsulfonyl)ethane]
read (ethylsulfonyl)ethane
Page 717, P-63.7, example 8 on this page. [modified 7 November 2018]
For 2 -(ethylperoxy)-1-methoxyethane
read 1-(ethylperoxy)-2-methoxyethane. [Deleted space from name and correct locants]
for (not [2-(methoxyethyl)peroxy]ethane;...
read {not [(2-methoxyethyl)peroxy]ethane;...
replace structure with:
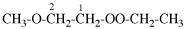
Page 717, P-63.7, example 9 on this page. [modified 5 June 2019]
For 1-methoxy 3-(methylthio)propane
read 1-methoxy-3-(methylthio)propane
replace structure with:
 Page 718, P-63.7, example 1 on this page . [corrected 27 February 2019]
Page 718, P-63.7, example 1 on this page . [corrected 27 February 2019]
For ... nor 1-methyl-2-[1-(methylsulfanyl)pent-1-en-1-yl]disulfane; ...
read ... nor methyl[1-(methylsulfanyl)pent-1-en-1-yl]disulfane; ...
Page 718, P-63.7, example 4 on this page. [corrected 26 September 2018]
Replace the structure with:
Page 719, P-63.8.1, paragraph 3. [modified 1 November 2023]
For The traditional names methoxide, ethoxide, proproxide, butoxide, phenoxide, and aminoxide, , for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, C6H5-O–, and H2N-O– are retained for use in general nomenclature and may be substituted in the same way as the corresponding alcohols. The traditional names isopropoxide and tert-butoxide for (CH3)CH-O– and (CH3)3C-O– also are retained but cannot be substituted.
read The traditional names methoxide, ethoxide, propoxide, butoxide and phenoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O– and C6H5-O–, are retained as preferred IUPAC names and aminoxide, H2N-O–, as a preselected name, and they may be substituted in the same way as the corresponding alcohols. The traditional name tert-butoxide for (CH3)3C-O– is also retained as a preferred IUPAC name but cannot be substituted. The traditional name isopropoxide for (CH3)2CH-O– is retained for general nomenclature but cannot be substituted.
Page 719, P-63.8.1, example 1.
For sodium methanolate (PIN)
read sodium methanolate
for sodium methoxide
read sodium methoxide (PIN)
Page 719, P-63.8.1, example 2.
For sodium propan-1-olate (PIN)
read sodium propan-1-olate
for sodium propoxide
read sodium propoxide (PIN)
Page 719, P-63.8.1, example 4. [modified 9 January 2017]
For llithium phenolate (PIN)
read lithium phenolate
for lithium phenoxide
read lithium phenoxide (PIN)
Page 719, P-63.8.2, example.
For 2,2′-spirobi[1,3,2]benzodioxagermole
read 2,2′-spirobi[[1,3,2]benzodioxagermole]
Page 720, P-63.8.3, example 1. (corrected 4 March 2020)
For HO-CH3-CH2-CH2-O– Na+
read HO-CH2-CH2-CH2-O– Na+
Page 722, P-64.1.2.1, example 2 on this page. [corrected 5 July 2018]
Replace structure with:
Page 722, P-64.1.2.2, example 1. (corrected 4 March 2020)
For methyl(dioxo)-λ5-phospane
read methyldi(oxo)-λ5-phospane
Page 723, P-64.2.1.1, last example
For 4-[(1E)-3-(dihydroxyphenyl)-3-oxoprop-1-en-1-yl]benzamide (PIN)
read 4-[(1E)-3-(2,4-dihydroxyphenyl)-3-oxoprop-1-en-1-yl]benzamide (PIN)
Page 723, P-64.2.1.2, lines 1/2. [corrected 11 March 2020]
For...acetone, 1,4-benzoquinone, naphthoquinone, and anthraquinone are retained...
read ...acetone and 1,4-benzoquinone are retained...
Page 723, P-64.2.1.2 last example on this page.
For 1-phenylethanone
read 1-phenylethan-1-one (PIN)
for acetophenone (PIN)
read acetophenone
Page 724, P-64.2.1.2, example 1 on this page. [corrected 11 March 2020]
Add (not benzoquinone)
Page 724, P-64.2.1.2, example 2 on this page. [corrected 11 March 2020]
Add (not naphthoquinone)
Page 724, P-64.2.1.2, example 3 on this page. [corrected 11 March 2020]
Add (not anthraquinone)
Page 724, P-64.2.1.2, example 5 on this page. [corrected 11 March 2020]
Delete as it is a duplicate of example 1 on page 723.
Page 724, P-64.2.1.3, example 1. [corrected 11 March 2020]
For acenaphthoquinone
read (not acenaphthoquinone)
Page 724, P-64.2.1.3, example 2. [corrected 11 March 2020]
For isoquinolone (1-isomer shown)
read [not isoquinolone (1-isomer shown)]
Page 725, P-64.2.1.3, example 3 on this page. [modified 26 January 2022]
For benzal
read [not benzil]
For quinolone (2-isomer shown)
read [not quinolone (2-isomer shown)]
Page 725, P-64.2.1.3, example 2 on this page. [corrected 11 March 2020]
For pyrrolidone (2-isomer shown)
read [not pyrrolidone (2-isomer shown)]
Page 725, P-64.2.1.3, example 3 on this page. [modified 11 March 2020]
For 1,2-diphenylethane-1,2-dione (PIN)
read diphenylethanedione (PIN)
for benzal
read [not benzal]
Page 725, P-64.2.1.3, example 4 on this page. [modified 11 March 2020]
For biacetyl
read [not biacetyl]
Page 725, P-64.2.1.3, example 5 on this page. [modified 11 March 2020]
For propiophenone
read [not propiophenone]
Page 726, P-64.2.2.1, example 2 on this page.
For 3-methylbutyl methyl ketone
read methyl 3-methylbutyl ketone
Page 726, P-64.2.2.1, last example on this page.
For 1,2-di(naphthalen-2-yl)ethane-1,2-dione (PIN)
read di(naphthalen-2-yl)ethanedione (PIN)
Page 728, P-64.2.2.2.2, last example.
For chrysene-1,3,6,8(2H,7H)-tetrone (PIN)
read pyrene-1,3,6,8(2H,7H)-tetrone (PIN)
for 1,2,3,6,7,8-hexahydrochrysene-1,3,6,8-tetrone
read 1,2,3,6,7,8-hexahydropyrene-1,3,6,8-tetrone
Page 728, P-64.2.2.2.3, lines1/2. [corrected 11 March 2020]
For ...The names 1,4-benzoquinone, naphthoquinone, and anthraquinone are retained for use...
read...The name 1,4-benzoquinone is retained for use...
Page 730, P-64.2.2.3, example 8. [corrected 4 September 2024]
For 1,3,6,8(2,5)-tetrafuranacyclododecaphan-11-en-2-one (PIN)
read (11Z)-1,4,7,10(2,5)-tetrafuranacyclododecaphan-11-en-2-one (PIN)
Page 732, P-64.2.2.4, example 3. (modified 4 March 2020)
For 2,2-dibromoethen-1-one
read dibromoethenone
Page 733, P-64.3.1, example 1 on this page.
For (1,2-dihydroquinolin-2-one)
read (not 1,2-dihydroquinolin-2-one)
Page 733, P-64.3.1, example 4 on page.
For pyrimidine-2,4,6(1H,3H,5H)-trione (PIN)
read pyrimidine-2,4,6(1H,3H,5H)-trione
add 1,3-diazinane-2,4,6-trione (PIN)
Page 733, P-64.3.1, example 5 on page.
For 1,3,5-triazine-2,4,6(1H,3H,5H)-trione (PIN)
read 1,3,5-triazine-2,4,6(1H,3H,5H)-trione
add 1,3,5-triazinane-2,4,6-trione (PIN)
Page 733, P-64.3.1, last example on page.
For 2′H,4′H-[2,3′-bipyranylidene]-2′,5(6H)-dione
read 2′H,4′H-[2,3′-bipyranylidene]-2′,5(6H)-dione
Page 734, P-64.3.1, last example.
For 2′H,4′H-[2,3′-bipyranylidene]-4′,6(5H)-dione (PIN)
read 2′H,4′H-[2,3′-bipyranylidene]-4′,6(5H)-dione (PIN)
Page 734, P-64.3.2, example 1.
For 1-(1-oxopropyl)piperidine)
read 1-(1-oxopropyl)piperidine
Page 734, P-64.3.2, example 4.. [corrected 12 May 2021]
For acetyl(trimethyl)silane
read acetyltri(methyl)silane
Page 736, P-64.4.2, example 1 on this page.
For 1λ4-thiophen-1-one (PIN)
read 1H-1λ4-thiophen-1-one (PIN)
for 1-oxo-1λ4-thiophene
read 1-oxo-1H-1λ4-thiophene
Page 736, P-64.4.2, example 2 on this page.
For 5λ6-thianthrene-5,5-dione (PIN)
read 5H-5λ6-thianthrene-5,5-dione (PIN)
for 5,5-dioxo-5λ6-thianthrene
read 5,5-dioxo-5H-5λ6-thianthrene
Page 737, P-64.5.1, example 1.
For 2-(2-oxocyclohexyl)propan-2-one
read 1-(2-oxocyclohexyl)propan-2-one
Page 738, P-64.5.2.1, example 1.
For 3-(2-oxopiperidin-3-yl)propan-2-one
read 1-(2-oxopiperidin-3-yl)propan-2-one
Page 738, P-64.5.2.1, example 2.
For (not 3-propionylphosphepan-2-one)
read 3-propionylphosphepan-2-one
Page 738, P-64.5.2.1, example 4.
For (not 1-propionyl pipeeridine)
read 1-propionylpiperidine [delete space in name]
Page 741, P-64.6.2, example 2 on this page. [corrected 7 November 2018]
For 1,1′-carbonothioyldi[pyridin-2(1H)-one] (PIN)
read 1,1′-carbonothioyldi(pyridin-2(1H)-one) (PIN)
for 1,1′-thiocarbonyldi[pyridin-2(1H)-one]
read 1,1′-thiocarbonyldi(pyridin-2(1H)-one)
Page 746, P-65.1.1.2.1, example 1.
For furoic acid (also 3- isomer)
read 2-furoic acid (also 3-isomer)
Page 747, P-65.1.1.2.2, example 3 on this page.
For naphthoic acid
read 2-naphthoic acid (also 1-isomer)
Page 747, P-65.1.1.2.2, end of list. [corrected 18 September 2019]
Add examples 1-3 of page 749, P-65.1.1.2.4 on this page
Page 748, P-65.1.1.2.4, example 1. [modified 11 March 2020]
For propiolic acid
read (not propiolic acid)
for but-2-ynoic acid (PIN)
read prop-2-ynoic acid (PIN)
Page 748, P-65.1.1.2.4, example 2. [modified 11 March 2020]
For isobutyric acid
read (not isobutyric acid)
for 3-methylpropanoic acid (PIN)
read 2-methylpropanoic acid (PIN)
Page 748, P-65.1.1.2.4, example 3. [corrected 11 March 2020]
For acetoacetic acid
read (not acetoacetic acid)
Page 748, P-65.1.1.2.4, example 4. [corrected 11 March 2020]
For anthranilic acid (1,2-isomer only)
read [not anthranilic acid (1,2-isomer only)]
Page 748, P-65.1.1.2.4, example 5. [modified 11 March 2020]
For benzilic acid
read [not benzilic acid]
for hydroxy(diphenyl)acetic acid (PIN)
read hydroxydi(phenyl)acetic acid (PIN)
Page 748, P-65.1.1.2.4, example 6. Move this example to P-65.1.1.2.2. [modified 3 March 2021]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Add 2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
for (HOOC-CH2)2-N-CH2-CH2-N-(CH2-COOH)2
read (HOOC-CH2)2N-CH2-CH2-N(CH2-COOH)2
Page 748, P-65.1.1.2.4, example 7. [corrected 11 March 2020]
For glycolic acid
read (not glycolic acid)
Page 748, P-65.1.1.2.4, example 8. [corrected 11 March 2020]
For glycoxylic acid
read (not glycoxylic acid)
Page 749, P-65.1.1.2.4, examples 1-3 on this page. [corrected 7 August 2019]
Delete these examples
Page 749, P-65.1.2.1, example 3. [corrected 24 October 2018]
Replace the structure with:
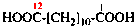
Page 749, P-65.1.2.1
Add the following example:
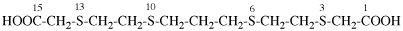
3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
Page 751, P-65.1.2.2.2. example 3 on this page. [corrected 24 October 2018]
For benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)-5-carboxylic acid
read [benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid
replace the structure with:
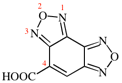
Page 751, P-65.1.2.2.3, example 1.
For 4-carboxy-1-methylpyridinium chloride
read 4-carboxy-1-methylpyridin-1-ium chloride
Page 752, P-65.1.2.2.3, example 1 on this page. [corrected 19 September 2018]
For 1-methyl-3-oxalobicyclo[2.2.2]octan-1-ium (PIN)
read 1-methyl-3-oxalo-1-azabicyclo[2.2.2]octan-1-ium (PIN)
Page 753, P-65.1.2.4, example 6.
For HO-CH2-CH2-O)2CH-COOH
read (HO-CH2-CH2-O)2CH-COOH
Page 754, P-65.1.2.4, example 1 on this page.
For 4-(hydroxysulfanyl)methyl]benzoic acid (PIN)
read 4-[(hydroxysulfanyl)methyl]benzoic acid (PIN)
Page 754, P-65.1.2.4, example 4 on this page. [corrected 26 September 2018]
Replace the structure with:
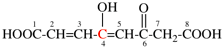
Page 754, P-65.1.2.4, example 5. [corrected 6 March 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 755, P-65.1.2.4, example 1 on this page. [corrected 6 March 2019]
For N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-ethane-1,2-diyldiglycine
read N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
Page 757 P-65.1.3.1.1, example on this page. [corrected 25 August 2016]
For (see P-65.1.3.1.1; P-65.1.5.2)
read (see P-65.1.5.2)
Page 757, P-65.1.3.2.1, example 1. [corrected 10 October 2018]
Replace the structure with:
H-C(=N-NH2)-OH
Page 760, P-65.1.3.4, example 1. [deleted 24 March 2021]
Delete this correction.
Page 760, P-65.1.3.4, example 2. [deleted 24 March 2021]
Delete this correction.
Page 761, P-65.1.4.1, example 1.
For (not performic acid)
read performic acid
Page 761, P-65.1.4.1, example 2.
For (not peracetic acid)
read peracetic acid
Page 761, P-65.1.4.1, example 4.
For (not perbenzoic acid)
read perbenzoic acid
Page 763, P-65.1.5.1, example 4. [corrected 9 January 2019]
For H{S,O}C-CH2-CH2-CH2-CH2-C{O/S}H
read H{S/O}C-CH2-CH2-CH2-CH2-C{O/S}H
Page 763, P-65.1.5.1, example 10.
For 4-ethanethioylbenzoic acid (PIN)
read 4-(ethanethioyl)benzoic acid (PIN)
Page 764, P-65.1.5.1, example 2 from top of page.
For sulfanyl(oxo)acetic acid (PIN)
read oxo(sulfanyl)acetic acid (PIN)
Page 764, P-65.1.5.1, example 3 from top of page.
For 4-[hydroxy(carbonothioyl)]pyridine-2-carboxylic acid (PIN)
read 4-(hydroxycarbonothioyl)pyridine-2-carboxylic acid (PIN)
Page 764, P-65.1.5.1, example 8 from top of page.
For N-hydroxycyclohexane-1-carboximidoselenoic acid
read N-hydroxycyclohexanecarboximidoselenoic acid
Page 764, P-65.1.5.1, last example.
For 3-amino-3-(ethylsulfanyl)prop-2-enedithioic acid (PIN)
read 3-amino-3-(ethylsulfanyl)prop-2-ene(dithioic acid) (PIN)
Page 765, P-65.1.5.2, example 4. [modified 5 June 2019]
For 3-amino-2,3-dioxopropanethoic (O,S)-acid (PIN)
read 3-amino-2,3-dioxopropanethoic acid (PIN)
for H2N-CO-CO-C(S/O)-OH
read H2N-CO-CO-C{O/S}H
Page 765, P-65.1.5.2, example 5.
For 3-(thiocarboxy)butanoic acid
read 4-(thiocarboxy)butanoic acid
Page 765, P-65.1.5.2, example 8. [corrected 19 September 2018]
For hydroxyl(sulfanylidene)acetic acid (PIN)
read hydroxy(sulfanylidene)acetic acid (PIN)
Page 765, P-65.1.5.2, example 10.
For 2-(thiocarboxy)benzenecarbothioic S-acid (PIN)
read 2-(thiocarboxy)benzene-1-carbothioic S-acid (PIN)
Page 765, P-65.1.5.2, example 11. [corrected 6 March 2019]
For (not 1-selenophthalic Se-acid)
read (not selenoterephthalic Se-acid)
Page 766, P-65.1.5.2, example on this page.
For ethanebis(dithioic) acid (PIN)
read ethanebis(dithioic acid) (PIN)
Page 766, P-65.1.5.3, example 5.
For –COS2H dithiocarboperoxic acid (preferred suffix; location of sulfur atom unknown)
read –COS2H dithiocarboperoxoic acid (preferred suffix; location of sulfur atom unknown)
Page 767, P-65.1.5.3, example 1 on this page. [corrected 26 September 2018]
Replace the structure with:
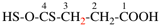
Page 767, P-65.1.5.3, example 2 on this page.
For 3-(dithiocarboperoxoyl)propanoic acid (PIN)
read 3-(dithiocarbonoperoxoyl)propanoic acid (PIN)
Page 767, P-65.1.5.3, example 3 on this page.
For (dithiocarboperoxoyl)formic acid (PIN)
read (dithiocarbonoperoxoyl)formic acid (PIN)
Page 767, P-65.1.5.3, last example.
For 4-[(hydroxysulfanyl)carbonyl]cyclohexanecarboxylic acid]
read 4-[(hydroxysulfanyl)carbonyl]cyclohexane-1-carboxylic acid
Page 768, P-65.1.6.2, example 1.
For 5-(phenylamino)-5-oxopentanoic acid
read 5-oxo-5-(phenylamino)pentanoic acid
Page 768, P-65.1.6.3, example 3. [corrected 18 September 2019]
Delete oxalaldehydic acid
Page 769, P-65.1.7.1, paragraph 2, line 3.
For ...‘1-oxopropyl’ and ‘iminoethyl’ for CH2-CH2-CO– and ...
read ...‘1-oxopropyl’ and ‘1-iminoethyl’ for CH3-CH2-CO– and ...
Page 770, P-65.1.7.2.2, example 3. [corrected 7 November 2018]
For (oxo)phenylmethyl
read oxo(phenyl)methyl
Page 770, P-65.1.7.2.2, example 4. [corrected 6 March 2019]
For dioxoethane-1,2-diyl
read dioxoethanediyl
Page 771, P-65.1.7.2.2, example 2 on this page. [corrected 28 October 2016]
For diiminoethane-1,2-diyl
read diiminoethanediyl
Page 771, P-65.1.7.2.3, last example. [corrected 5 June 2019]
For bis(sulfanylidene)ethane-1,2-diyl
read bis(sulfanylidene)ethanediyl
Page 772, P-65.1.7.2.4, example 1.
Delete oxaldehydoyl
Page 772, P-65.1.7.3.1, line 2. [corrected 25 August 2016]
For ...nomenclature (see P-65-4.3.2); substitution...
read ...nomenclature (see P-65.1.1.2); substitution...
Page 774, P-65.1.7.3.3, example 3.
For 2-sulfanyl-2-sulfanylideneethanethioyl (preferred prefix)
read sulfanyl(sulfanylidene)ethanethioyl (preferred prefix)
Page 774, P-65.1.7.4.1, example 2. [corrected 6 March 2019]
For 1,10-dioxodecanediyl
read 1,10-dioxodecane-1,10-diyl
Page 775, P-65.1.7.4.1, example 1 on this page. [corrected 9 January 2019]
For 1-sulfanylidene propyl
read 1-sulfanylidenepropyl [delete space]
Page 775, P-65.1.7.4.2, example 3.
Move carbohydrazonoyl to example 4 (part of name)
delete (not dithiophthaloyl)
delete 1,4-phenylenebis(sulfanylidenemethylene)
delete 1,4-phenylenebis(thioxomethylene)
replace the structure with:
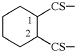
Page 775, P-65.1.7.4.2, example 4.
Move carbohydrazonoyl from under example 3 to under the rest of the first name for example 4.
for 1-methylcyclopentyl(hydrazinylidene)methyl
read hydrazinylidene(1-methylcyclopentyl)methyl
Page 775, P-65.1.7.4.2, example 6. [corrected 19 September 2018]
For hexane-2,3,5-triyltris(thioxoemethylene)
read hexane-2,3,5-triyltris(thioxomethylene)
replace the structure with:
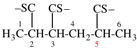
Page 776, P-65.1.7.4.3, example 2. (modified 27 November 2019)
For 1,4-phenylenebis(sulfanylidenemethylene)
read 1,2-phenylenebis(sulfanylidenemethylene)
for 1,4-phenylenebis(thioxomethylene)
read 1,2-phenylenebis(thioxomethylene)
Page 778, P-65.2, example. [corrected 6 March 2019]
For 2,4,6,8-tetraoxanonanedioic acid (PIN)
read 3,5,7-trioxo-2,4,6,8-tetraoxanonanedioic acid (PIN)
Page 778, P-65.2.1.1, example 2.
Replace structure with:
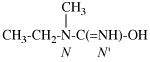
Page 778, P-65.2.1.1, example 3.
For 2-hydroxypropyl N-(2-aminoethyl)carbamate (PIN)
read 2-hydroxypropyl (2-aminoethyl)carbamate (PIN)
Page 782, P-65.2.1.5, example 4 on this page. [corrected 6 March 2019]
For aminocarbonimidoyl
read C-aminocarbonimidoyl
Page 783, P-65.2.1.7, example 4. [corrected 6 March 2019]
For (3) (dithiohydroperoxycarbonyl)oxy
read (3) [(dithiohydroperoxy)carbonyl]oxy
Page 784, P-65.2.2, example 1. [corrected 19 September 2018]
For carbononitridothioic
read carbononitridothioic acid
Page 785, P-65.2.2, example 1 on this page. [corrected 18 September 2019]
For carbonitridoylacetic acid
read carbononitridoylacetic acid
Page 785, P-65.2.2, example 2 on this page. [corrected 6 March 2019]
For 3-carbonitridothioylpropanoic acid
read 3-(carbononitridoylthio)propanoic acid
Page 787, P-65.2.3.1.2.3, example 1.
For [(thiocarboxy)oxy]formothioic S-acid (PIN)
read [(thiocarboxy)oxy]methanethioic S-acid (PIN)
Page 787, P-65.2.3.1.2.3, example 2. [modified 31 October 2018]
For [(thiocarboxy)oxy]formothioic O-acid (PIN)
read [(thiocarboxy)oxy]methanethioic O-acid (PIN)
Page 787, P-65.2.3.1.3, example 2. [modified 31 October 2018]
For 1-isocyanato-2-imidodicarbonic acid
read 2-imido-1-isocyanatodicarbonic acid
Page 788, P-65.2.3.1.5, example 2. [corrected 7 November 2018]
For {not (dithiocarboxy)sulfanyl]thioformyl}
read {not [(dithiocarboxy)sulfanyl]thioformyl}
Page 788, P-65.3.0, example 4. [corrected 19 September 2018]
For R-SO2H seleninic acid
read R-SeO2H seleninic acid
Page 790, P-65.3.1.3, example 1. [modified 24 June 2020]
For propane-1-sulfonoselenoic acid (PIN)
read propane-1-sulfinoselenoic acid (PIN)
Page 790, P-65.3.1.4, line 2. [corrected 10 October 2018]
For ...such as ‘sulfinimidic acid’ for –S(O)(=NH)-OH, ...
read ...such as ‘sulfinimidic acid’ for –S(=NH)-OH, ...
Page 791, P-65.3.1.4, example 4 on this page. [corrected 26 September 2018]
Replace the structure with:
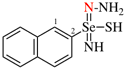
Page 791, P-65.3.1.5, example 2.
For N-hydroxypropanesulfinamide (PIN)
read N-hydroxypropane-1-sulfinamide (PIN)
Page 792, P-65.3.2.1, example 4 on this page.
Replace structure with:
H{S/O}S-CH2-CH2-SO2-OH
Page 792, P-65.3.2.1, example 5 on this page. [modified 6 March 2019]
For 2-trithiosulfobenzoic acid (PIN)
read 2-(trithiosulfo)benzoic acid (PIN)
for sulfanylsulfonodithioyl
read 2-(sulfanylsulfonodithioyl)benzoic acid
Page 797, P-65.5.1.1, example 3.
Replace structure with:
Page 797, P-65.5.1.1, example 4.
For cyclohexanecarbonimidoyl chloride (PIN)
read cyclohexanecarboximidoyl chloride (PIN)
Page 797, P-65.5.1.1, example 6.
For cyclohexanecarbonothioyl chloride (PIN)
read cyclohexanecarbothioyl chloride (PIN)
Page 798, P-65.5.2.1, example 3. [corrected 31 October 2018]
For butanedioyl isocyanate isocyanide (PIN)
read butanedioyl isocyanide isothiocyanate (PIN)
Page 799, P-65.5.3.2, example 3.
For imidodicarbonic dichloride (PIN)
read 2-imidodicarbonic dichloride (PIN)
Page 799, P-65.5.4 (2), line 2. (corrected 1 December 2021)
For ...appropriate bivalent acyl group...
read ...appropriate divalent acyl group...
Page 800, P-65.5.4, example 2. [corrected 24 June 2020]
For (1) 2-(carbonochloridoyl)benzoic acid (PIN)
read (1) 2-carbonochloridoylbenzoic acid (PIN)
for (2) 2-chlorocarbonylbenzoic acid
read (2) 2-(chlorocarbonyl)benzoic acid
Page 800, P-65.5.4, example 4. [modified 24 June 2020]
For (1) 2-(carbonocyanidothioyl)benzoyl chloride (PIN)
read (1) 2-carbonocyanidothioylbenzoyl chloride (PIN)
for (2) 2-(cyanocarbonothioyl)benzoyl chloride
read (2) 2-(cyanocarbothioyl)benzoyl chloride
replace structure with:
Page 800, P-65.5.4, example 5. [modified 24 June 2020]
For 4-(2-isocyanato-2-oxoethyl)benzenecarbothioyl cyanide (PIN)
read (4-carbonocyanidothioylphenyl)acetyl isocyanate (PIN)
add [not 4-(2-isocyanato-2-oxoethyl)benzenecarbothioyl cyanide, acetyl is senior to carbothioyl]
replace the structure with:
Page 800, P-65.5.4, example 6. [corrected 24 June 2020]
For 2-(carbonocyanidoyl)-5-methylbenzoyl chloride (PIN)
read 2-carbonocyanidoyl-5-methylbenzoyl chloride (PIN)
Page 800, P-65.5.4, example 7. [corrected 28 October 2016]
For (3) 4-bromo-3,4-dioxobutanoic acid
read (3) 4-bromo-3,4-dioxobutanoic acid (PIN)
Page 800, P-65.5.4, example 8. [corrected 24 June 2020]
For (1) 3-[(carbonobromidoyl)oxy]-3-oxopropanoic acid (PIN)
read (1) 3-(carbonobromidoyloxy)-3-oxopropanoic acid (PIN)
Page 801, P-65.6.2.1, example 2.
For sodium propanedithioate (PIN)
read sodium propane(dithioate) (PIN)
Page 802, P-65.6.2.2, example.
For 3,3′-spirobi[[2,4,3]benzodioxaplumbepin]-1,1′,5,5′-tetrone (PIN)
read 3,3′-spirobi[[2,4,3]benzodioxaplumbepine]-1,1′,5,5′-tetrone (PIN)
Page 802, P-65.6.2.3.1, example 1. [corrected 28 October 2016]
For (1) potassium 6-carboxyheptanoate (PIN)
read (1) potassium 6-carboxyhexanoate (PIN)
Page 803, P-65.6.2.3.1, example 2 on this page. [corrected 28 October 2016]
For (HOOC-CH2-CH2-COO–)3 Sb3
read (HOOC-CH2-CH2-COO–)3 Sb3+
for (2) antimony tris(hydrogen butanoate)
read (2) antimony tris(hydrogen butanedioate)
Page 804, P-65.6.3.2.1, example 2. [corrected 28 October 2016]
For CH3-O-CO-CH2-CH2-CO-O CH2-CH3
read CH3-O-CO-CH2-CH2-CO-O-CH2-CH3
Page 804, P-65.6.3.2.2.
Rewrite the second sentence in the box as follows: ‘The bi- or polyvalent functional class name is cited as the organyl (alkanediyl, arylene, etc.) group cited immediately before the name of the acid component... ’
Page 805, P-65.6.3.2.2, example 1 on this page. [corrected 11 December 2023]
For 1,4-phenylene bis(methyl propanedioate)
read 1,4-phenylene di(methyl propanedioate)
Page 805, P-65.6.3.2.3, lines 4/5. [corrected 25 August 2016]
For ...and ‘alkyloxy(alkanyl)...oxo’ or ‘alkyl(alkanyl)oxycarbonyl’ for the group –CO-OR′.
read ...and ‘alkoxy...oxo’, ‘(alkyloxy)...oxo’, ‘(alkanyloxy)...oxo’, ‘alkoxycarbonyl’, ‘(alkyloxy)carbonyl’ or ‘(alkanyloxy)carbonyl’ for the group –CO-OR′.
Page 805, P-65.6.3.2.3, example 1. (modified 4 March 2020)
For [2-(ethoxycarbonyl)ethyl]-N,N,N-trimethylammonium bromide
read [2-(ethoxycarbonyl)ethyl]tri(methyl)ammonium bromide
For [3-ethoxy-3-oxopropyl]-N,N,N-trimethylazanium bromide
read [3-ethoxy-3-oxopropyl]tri(methyl)azanium bromide
Page 805, P-65.6.3.2.3, example 2. [corrected 25 August 2016]
For 3-[(phenylcarbony)oxy]propanoic acid
read 3-[(phenylcarbonyl)oxy]propanoic acid
Page 805, P-65.6.3.2.3, first example.
For 3-ethoxy-N,N,N-trimethyl-3-oxopropanaminium bromide
read 3-ethoxy-N,N,N-trimethyl-3-oxopropan-1-aminium bromide
Page 806, P-65.6.3.2.3, last example.
This example is an ester and belongs in P-65.6.3.3.1.
Add S-(2-cyanoethyl) cyclohexanesulfinothioate (PIN)
for 3-[(cyclohexanesulfinyl)sulfanyl]propanenitrile (PIN)
read {not 3-[(cyclohexanesulfinyl)sulfanyl]propanenitrile;
for 3-[(cyclohexylsulfinyl)sulfanyl]propanenitrile; see P-65.4.1 for naming acyl groups derived from acids]
read nor 3-[(cyclohexylsulfinyl)sulfanyl]propanenitrile; see P-65.4.1for naming acyl groups derived from acids}
Page 807 P-65.6.3.3.1, example 2.
Following P-16.5.2.6 replace the structure with:
CH3-[CH2]6-CO-O-C(CH3)3
Page 808, P-65.6.3.3.2.1, last example. [corrected 25 August 2016]
For ethoxy... is alphabetically preferred to ethyl...)
read butanedioate is preferred to propandioate)
Page 809, P-65.3.3.2.2.1 (2), lines 2/3. [corrected 28 May 2024]
For ...representing the ‘hydroxylic’ are exactly...
read ...representing the ‘hydroxylic’ component are exactly...
Page 809, P-65.6.3.3.2.2.1, example 1. [corrected 28 May 2024]
For (2) 3,3′-oxydi(methyl benzoate)
read (2) 3,3′-oxybis(methyl benzoate)
Page 809, P-65.6.3.3.2.2.1, example 3. [modified 18 December 2023]
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylenedibutanedioate (PIN) [cancelled 28 May 2024]
for (2) butanebis(oxy-2,1-phenylene) di(methyl butanedioate)
read (2) butanebis(oxy-2,1-phenylene)di(methyl butanedioate)
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 28 May 2024]
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene)dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 7 November 2018]
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylene)dibutanedioate (PIN)
Page 809, P-65.6.3.3.2.2.1, example 3. [modified 9 October 2024]
For (2) butanedioylbis(oxy-2,1-phenylene) di(methyl butanedioate)
read (2) butanedioylbis(oxy-2,1-phenylene) bis(methyl butanedioate)
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 7 November 2018]
For (not bis[2-(4-methoxy-4-oxobutanoyl)oxy]phenyl butanedioate; ...
read (not bis{2-[(4-methoxy-4-oxobutanoyl)oxy]phenyl} butanedioate; ...
Page 810, P-65.6.3.3.2.2.2, example.
For methyl 6-chloro-3-[3-(ethoxycarbonyl)phenoxy]benzoate (PIN)
read methyl 2-chloro-5-[3-(ethoxycarbonyl)phenoxy]benzoate (PIN)
Replace the structure with:
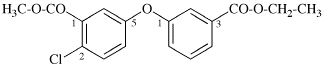
The published structure numbers corresponds to the multiplicative name
Page 810, P-65.3.3.3.1, example 4. [modified 24 June 2020]
For 3,3′-(1,2-phenylene)dipropyl diacetate [PIN; see see P-15.3.0(2) and P-51.3.1)
read 1,2-phenylenedi(propane-3,1-diyl) diacetate [PIN; see P-15.3.0 (2) and P-51.3.1]
Page 811, P-65.6.3.3.3.2, example 3.
Replace structure with:
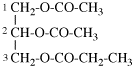
Page 811, P-65.6.3.3.3.2, last example. [corrected 28 October 2016]
For (2S)-propane-1,2,3-triyl 2-acetate 1-hexadecanoate 3-[(9Z)-octadec-9-enoate] (PIN)
read (1) propane-1,2,3-triyl 2-acetate 1-hexadecanoate 3-[(9Z)-octadec-9-enoate] (PIN)
for (2S)-2-(acetyloxy)-3-(hexadecanoyloxy)propyl (9Z)-octadec-9-enoate
read (2) 2-(acetyloxy)-3-(hexadecanoyloxy)propyl (9Z)-octadec-9-enoate
replace structure with:
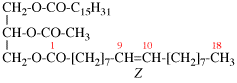
Note deletion of extra closing parenthesis.
Page 812, P-65.6.3.3.4.1, example. [corrected 18 December 2023]
For ethane-1,2-diyl bis(methyl butanedioate)
read ethane-1,2-diyl di(methyl butanedioate) [cancelled 28 May 2024]
Page 812, P-65.6.3.3.4.1, example. [corrected 28 May 2024]
For ethane-1,2-diyl di(methyl butanedioate)
read ethane-1,2-diyl bis(methyl butanedioate)
Page 812, P-65.6.3.3.4.2, example 1. [corrected 6 March 2019]
Replace the structure with:
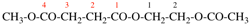
Page 812, P-65.6.3.3.4.2, example 2.
Following P-16.5.2.6 replace the structure with:
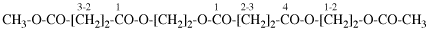
Page 813, P-65.6.3.3.4.2, example 1 on this page.
For [not phenyl 3-(phenoxycarbonyl)benzoate; ...
read [not 3-(phenoxycarbonyl)phenyl benzoate; ...
Page 813, P-65.6.3.3.4.2, example 2 on this page. (modified 4 March 2020)
For 3,3′-{2-[3-(formyloxy)propyl]cyclohexane-1,1-diyl}dipropyl diacetate (PIN)
read 2-[3-(formyloxy)propyl]cyclohexane-1,1-diyldi(propane-3,1-diyl) diacetate (PIN)
Page 813, P-65.6.3.3.4.2, example 3 on this page. [corrected 6 March 2019]
For (not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate; butanedioic acid is preferred to propanoic acid
read (not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate; butanedioic acid is preferred to propanedioic acid)
Page 813, P-65.6.3.3.4.2, example 4 on this page. [corrected 5 July 2018]
For the organyl groups in the PIN name are lower alphanumerically; see P-14.5
read 3-(methoxycarbonyl)phenyl is alphabetically before 2-methoxy-2-oxoethyl; see P-14.5
Page 813, P-65.6.3.3.4.2, example 5 on this page.
For bis[3-(methoxycarbonyl)phenyl butanedioate
read bis[3-(methoxycarbonyl)phenyl] butanedioate
Page 813, P-65.6.3.3.4.2, replace structure of example 5 on this page. [corrected 26 January 2022]
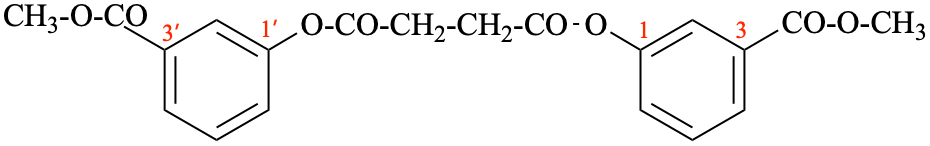
Page 814, P-65.6.3.3.4.2, example 1 on this page (example 10 of this section). [corrected 9 April 2025]
For
1-{2-[2-(acetyloxy)-1-(formyloxy)ethyl]cyclohexyl}-2-(propanoyloxy)ethyl butanoate (PIN)
read 1-{2-[2-(acetyloxy)-1-(formyloxy)ethyl]cyclohexyl}ethane-1,2-diyl 1-butanoate 2-propanoate (PIN)
Page 814, P-65.6.3.3.4.2, example 2 on this page. [corrected 26 September 2018]
Replace the structure with:
Page 814, P-65.6.3.3.4.2, example 4 on this page. [corrected 19 September 2018]
For (not 2-{2-[(acetyloxy)methyl]phenyl}ethyl acetate; ...
read (not 2-{2-[(acetyloxy)ethyl]phenyl}methyl acetate; ...
Page 814, P-65.6.3.3.4.2, example 5 on this page. [corrected 28 October 2016]
Delete this example [Duplicate of example 3].
Page 814, P-65.6.3.3.4.2, example 6 on this page. [corrected 28 October 2016]
Delete this example [Duplicate of example 4].
Page 815, P-65.6.3.3.4.3, example 2. [corrected 28 October 2016]
For dimethyl ethane-1,2-diylbis(carbonyloxyethyl) dibutanedioate)
read dimethyl ethane-1,2-diylbis(carbonyloxyethane-2,1-diyl) dibutanedioate
Page 815, P-65.6.3.3.4.3, example 3. [corrected 28 October 2016]
For dimethyl 6,8-dioxo-2,5,9,12-tetraoxatridecane-1,13-diyl dipropanedioate (PIN)
read dimethyl 6,8-dioxo-2,5,9,12-tetraoxatridecane-1,13-diyl dipropanedioate
For dimethyl propanedioylbis(oxyethane-2,1-diyloxymethylene dipropanedioate)
read dimethyl propanedioylbis(oxyethane-2,1-diyloxymethylene) dipropanedioate
add dimethyl 3,10,12,19-tetraoxo-4,6,9,13,16,18-hexaoxahenicosane-1,21-dioate (PIN)
replace the structure with:
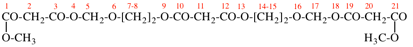
Page 815, P-65.6.3.3.4.3, example 4. [corrected 28 October 2016]
For 2-({[(methoxycarbonyl)acetyl]oxy}ethyl) 3,5,9,11-tetraoxo-2,6,8,12-tetraoxatridecan-1-yl butanedioate (PIN)
read 2-{[(methoxycarbonyl)acetyl]oxy}ethyl 3,5,9,11-tetraoxo-2,6,8,12-tetraoxatridecan-1-yl butanedioate
add dimethyl 3,7,9,13,16,21-hexaoxo-4,6,10,12,17,20-hexaoxatricosane-1,23-dioate (PIN)
replace the structure with:
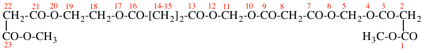
Page 816, P-65.6.3.3.4.4, example 1.
Delete [(not 2-{2-[(acetyloxy)methyl]phenyl}ethyl acetate‘; the ethyl chain is senior to the methyl chain’]
Page 816, P-65.6.3.3.5, example 2. [corrected 24 October 2018]
Replace the structure with:
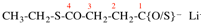
Page 817, P-65.6.3.3.5, example 1 on this page. (modified 4 March 2020)
For (1) potassium 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate (PÏN)
read (1) potassium hydrogen 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate (PIN)
for (2) potassium 1-ethyl hydrogen 2-hydroxypropane-1,2,3-tricarboxylate
read (2) potassium 3-ethyl hydrogen 2-hydroxypropane-1,2,3-tricarboxylate
for (2) potassium 5-ethyl hydrogen citrate
read (2) potassium 3-ethyl hydrogen citrate
add (the anionic –OOC– group is preferred to the ester group CH3-CH2-O-CO–)
Page 817, P-65.6.3.3.5, example 2 on this page.
For 6-chloro-2-(ethoxycarbonyl)benzoic acid (PIN)
read 2-chloro-6-(ethoxycarbonyl)benzoic acid (PIN)
replace the structure with:
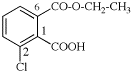
Page 817, P-65.6.3.3.5, example 3 on this page.
For 3-chloro-2-(ethoxycarbonyl)chlorobenzoic acid (PIN)
read 3-chloro-2-(ethoxycarbonyl)benzoic acid (PIN)
Page 819, P-65.6.3.3.7.1, example 6 on this page. [corrected 24 June 2020]
For 2-[(propanimidoyl)selanyl]benzene-1-carboximidic acid (PIN)
read 2-(propanimidoylselanyl)benzene-1-carboximidic acid (PIN)
Page 819, P-65.6.3.3.7.1, example 8 on this page. [corrected 24 October 2018]
Replace the structure with:

Page 819, P-65.6.3.3.7.1, example 9 on this page. [corrected 24 October 2018]
Replace the structure with:
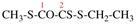
Page 819, P-65.6.3.3.7.1, last example on page.
For methyl (ethylsulfanyl)(sulfanylidene)ethanethioate (PIN)
read S-methyl (ethylsulfanyl)(sulfanylidene)ethanethioate (PIN)
Page 820, P-65.6.3.3.7.1, example on this page. [corrected 28 October 2016]
For O-methyl 2-{4-[(2-methoxy-2-oxoethanethioyl)oxy]phenoxy}-2-oxoethanethioate (PIN)
read [not O-methyl (4-{[methoxy(oxo)ethanethioyl]oxy}phenoxy)(oxo)ethanethioate; a carboxylic acid is preferred to a thiocarboxylic acid]
for [not methyl 2-{4-[(2-methoxy-2-sulfanylideneacetyl)oxy]phenoxy}-2-sulfanylideneacetate; the PIN is lower in alphanumerical order)
read [not methyl (4-{[methoxy(sulfanylidene)acetyl]oxy}phenoxy)(sulfanylidene)acetate; the PIN is lower in alphabetical order]
Add 4-{[methoxy(oxo)ethanethioyl]oxy}phenyl methoxy(sulfanylidene)acetate (PIN)
Page 820, P-65.6.3.3.7.2.1, example 1.
For 1-S-methyl 3-ethyl 1-thiodicarbonate (PIN)
read 3-ethyl 1-S-methyl 1-thiodicarbonate (PIN)
Page 820, P-65.6.3.3.7.2.2, example 1.
For 2-{[2-methylsulfanyl-1-oxo-2-sulfanylideneethane-1,2-diyl]oxy}ethanedithoic acid (PIN)
read {[(methylsulfanyl)(sulfanylidene)acetyl]oxy}ethane(dithioic acid) (PIN)
Page 820, P-65.6.3.3.7.2.2, example 2.
For S-ethyl 3-(cyanosulfanyl)propanethioate (PIN)
read S-ethyl 3-(thiocyanato)propanethioate (PIN)
Page 821, P-65.6.3.4.1, example 5.
For (CH3-CO-O)3-B
read (CH3-CO-O)3B
Page 821, P-65.6.3.4.1, example 7. [corrected 24 July 2019]
For O-acetyl-N,N-dimethylhydroxylamine (PIN)
read O-acetyl-N,N-dimethylhydroxylamine
add 1-[(dimethylamino)oxy]ethan-1-one (PIN)
Page 821, P-65.6.3.4.2, example.
For [1-(methylperoxy)sulfanyl]-butan-1-one
read 1-[(methylperoxy)sulfanyl]butan-1-one (PIN)
for (not S-methylperoxyl propanethioate
read (not S-methylperoxyl butanethioate)
Page 822, P-65.6.3.5.1, example 2.
For 1-oxadodecan-2-one (PIN)
read 1-oxacyclododecan-2-one (PIN)
for dodecano-12-lactone
read undecano-11-lactone
Page 823, P-65.6.3.5.1, example 2 on this page. [corrected 6 March 2019]
For 2-oxohexahydro-2H-benzooxete-5,6-dicarboxylic acid
read 2-oxohexahydro-2H-benzoxete-5,6-dicarboxylic acid
Page 824, P-65.6.3.5.2, example 1.
For 2λ6-naphtho[1,8-cd][1,2]oxathiole-2,2-dione (PIN)
read 2H-2λ6-naphtho[1,8-cd][1,2]oxathiole-2,2-dione (PIN)
Page 824, P-65.6.3.5.2, example 3.
Move pentane-2,5-sultone to example 2
Page 824, P-65.6.3.5.2, example 3. [corrected 9 January 2019]
For 1,2λ4-oxathiolan-2-thione (PIN)
read 1,2λ4-oxathiolane-2-thione (PIN)
Page 825, P-65.6.3.5.4, example 3. [corrected 24 June 2020]
For (not 3,4-dihydrobenzo[f]dioxocine-1,6-dione)
read (not 3,4-dihydrobenzo[f][1,4]dioxocine-1,6-dione)
Page 825, P-65.6.3.5.4, example 4.
For 3,4,6a,7,10,11,14,14a-octahydro[1,4]dioxocino][2,3-c][1,6]dioxecine-2,5,9,12-tetrone (PIN)
read octahydro[1,4]dioxocino[2,3-c][1,6]dioxecine-2,5,9,12-tetrone (PIN)
Page 826, P-65.6.3.5.4, example on this page.
For 3,4,6a,7a,10,11,14,14a-octahydro[1,5]dioxonino[3,2-b][1,5]dioxonine-2,5,9,12-tetrone (PIN)
read octahydro[1,5]dioxonino[3,2-b][1,5]dioxonine-2,5,9,12-tetrone (PIN)
Page 827, P-65.7.1, example 7. [corrected 9 January 2019]
For (Cl-CH2-CO-O)2O
read (Cl-CH2-CO)2O
for chloroacetic anhydride (PIN)
read chloroacetic anhydride
add bis(chloroacetic) anhydride (PIN)
Page 827, P-65.7.1, example 8. [modified 9 January 2019]
For Cl-CH2-CH2-SO)2O
read (Cl-CH2-CH2-SO)2O
for 2-chloroethane-1-sulfinic anhydride (PIN)
read 2-chloroethane-1-sulfinic anhydride
add bis(2-chloroethane-1-sulfinic) anhydride (PIN)
Page 828, P-65.7.2, example 3 on this page. [corrected 10 October 2018]
For HO-BH-O-CO-CH3
read (HO)2B-O-CO-CH3
Page 828, P-65.7.3, example 3. [corrected 6 March 2019]
For 4-chlorocyclohexane-1-carbothioic thioanhydride (PIN)
read bis(4-chlorocyclohexane-1-carbothioic) thioanhydride (PIN)
Page 829, P-65.7.4, example 2.
For acetic thioperoxy anhydride (PIN)
read acetic thioperoxyanhydride (PIN) [delete space in name]
Page 830, P-65.7.5.1, example 1. [modified 6 March 2019]
For 1,1′-(trioxidanediyl)di(ethan-1-one)
read 1,1′-trioxidanediyldi(ethan-1-one)
Replace the structure with:
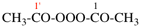
Page 830, P-65.7.5.1, example 3. [corrected 6 March 2019]
For 1,1′-(dithioxanediyl)di(ethan-1-one)
read 1,1′-dithioxanediyldi(ethan-1-one)
Page 830, P-65.7.5.1, example 4.
For {not 1-[(acetylsulfanyl)peroxy)]ethan-1-one;
read {not 1-[(acetylsulfanyl)peroxy]ethan-1-one;
Page 831, P-65.7.6.2, last example on this page.
For 4-[4-(acetyloxy)-4-oxobutanoic] 2-methyl-4-oxobutanedioic 1-propanoic dianhydride
read 4-[4-(acetyloxy)-4-oxobutanoic] 2-methylbutanedioic 1-propanoic dianhydride
Page 832, P-65.7.6.3, example 2. [corrected 31 October 2018]
For 6-acetic 3-[4-(acetyloxy)-4-oxo-2-methylbutanoic 2-propanoic naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
read 6-acetic 3-[4-(acetyloxy)-2-methyl-4-oxobutanoic] 2-propanoic naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
Page 832, P-65.7.6.3, example 3. [corrected 16 June 2021]
For P,P′-diacetic P,P′-dipropanoic ethane-1,2-diylbis(phosphonic) tetraanhydride (PIN)
read P,P′-diacetic P,P′-dipropanoic (ethane-1,2-diyl)bis(phosphonic) tetraanhydride (PIN)
Page 833, P-65.7.6.3, example 1 on this page. [corrected 31 October 2018]
For 5-(acetyloxy)-5-oxopentanoic 6-(butanoyloxy)-6-oxohexanoic 4-(propanoyloxy)-4-oxobutanoic phosphoric trianhydride
read 5-(acetyloxy)-5-oxopentanoic 6-(butanoyloxy)-6-oxohexanoic 4-oxo-4-(propanoyloxy)butanoic phosphoric trianhydride (PIN)
Page 833, P-65.7.6.4.2, example 1.
Remove second name
Page 834, P-65.7.6.4.3, example 3.
For acetic 4-(ethanethioylsulfanyl)-4-sulfanylidenebutanethioic anhydride (PIN)
read acetic 4-[(ethanethioyl)sulfanyl]-4-sulfanylidenebutanethioic anhydride (PIN)
Page 834, P-65.7.6.4.3, example 4.
For acetic butanedioic 4-(acetylsulfanyl)-4-oxobutanoic anhydride (PIN)
read acetic butanedioic 4-oxo-4-(propanoylsulfanyl)butanoic dianhydride (PIN)
Page 837, P-65.7.7.3 (2), line 1/2. [corrected 6 March 2019]
For ...‘dianhydride’ or ‘thioanhydride’, thiodianhydride, etc., in the ...
read ...‘dianhydride’ or ‘thioanhydride’, ‘bis(thioanhydride)’, etc., in the ...
Page 837, P-65.7.7.3, example 1. [corrected 6 March 2019]
For (1) hexahydro-1H-2-benzothiophene-1,3(4H)-dione (PIN)
read (1) hexahydro-2-benzothiophene-1,3-dione (PIN)
for hexahydro-1H-benzo[c]thiophene-1,3(4H)-dione
read hexahydrobenzo[c]thiophene-1,3-dione
Page 838, P-65.7.7.3, example 1 on this page. [corrected 19 September 2018]
For (1) hexhydro-1H-benzopyran-1,3(4H)-dithione (PIN)
read (1) hexahydro-1H-benzopyran-1,3(4H)-dithione (PIN)
Page 838, P-65.7.7.3, example 3 on this page. [corrected 6 March 2019]
For (1) 5,7-disulfanylidene-5,7-dihydro-1H, 3H-thieno[3,4-f][2]benzofuran-1,3-dione (PIN)
read (1) 5,7-bis(sulfanylidene)-5,7-dihydro-1H, 3H-thieno[3,4-f][2]benzofuran-1,3-dione (PIN)
for (2) 2-benzothiophene-5,6-dicarboxylic anhydride.
read (2) 1,3-bis(sulfanylidene)-1,3-dihydro-2-benzothiophene-5,6-dicarboxylic anhydride
for isobenzothiofuran-5,6-dicarboxylic anhydride
read 1,3-dithioxo-1,3-dihydroisobenzothiophene-5,6-dicarboxylic anhydride
Page 838, P-65.7.7.3, example 4 on this page. (modified 4 March 2020)
For (1) 3b,6a,7,7a- tetrahydro-1H-cyclopenta[1,2-c:3,4-c′]dithiophene-1,3,4,6(3aH)-tetrone (PIN)
read (1) tetrahydro-1H-cyclopenta[1,2-c:3,4-c′]dithiophene-1,3,4,6(3aH)-tetrone (PIN)
Page 839, P-65.7.8.1 example 1. [corrected 9 January 2019]
For bis(2-chloroethanesulfinic) acid
read bis(2-chloroethane-1-sulfinic) acid
Page 840, P-66.1.0, lines 3-5. [corrected 26 January 2022]
For ...three acyl groups on a single nitrogen atom are generically included and may be designated as primary, secondary, and tertiary amides, respectively.
read ...three acyl groups on a single nitrogen atom are generically included.
Page 841, P-66.1.1.1.1.3, example 3.
Replace structure with:
Page 843, P-66.1.1.3.1.1, line 6. [corrected 13 March 2019]
For ... for example, N1, N′1, etc. (see ...
read ... for example, N1, N3, etc. (see ...
Page 844, P-66.1.1.3.1.1, example 1. [corrected 26 January 2022]
For N,N-dimethylformamide (PIN)
read dimethylformamide (PIN)
Delete dimethylformamide
Page 844, P-66.1.1.3.1.1, example 2. [corrected 10 October 2018]
Replace the structure with:

Page 844, P-66.1.1.3.1.1, example 3. [corrected 26 September 2018]
Replace the structure with:
Page 844, P-66.1.1.3.1.1, example 5. [corrected 19 September 2018]
For N,N-diethyl-2-furanamide
read N,N-diethyl-2-furamide
Page 845, P-66.1.1.3.2, lines 2/3. [corrected 24 February 2021]
For The suffixes ‘hydroxamic acid’ and ‘carbohydroxamic acid’ are no longer recommended.
read The suffixes ‘hydroxamic acid’ and ‘carbohydroxamic acid’ may be used in general nomenclature.
Page 845, P-66.1.1.3.1.2, example 2.
Replace structure with:
Page 845, P-66.1.1.3.2, line 3. [modified 24 March 2021]
For ...acid’ are no longer recommended.
read ...acid’ may be used in general nomenclature.
Page 845, P-66.1.1.3.3, example 1.
Replace structure with:
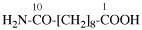
Page 847, P-66.1.1.3.5, example 2. [corrected 26 September 2018]
Replace the structure with:
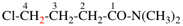
Page 847, P-66.1.1.3.5, last example.
For [ 2,2′-ethane-1,2-diylbis(azanediyl)]di(cyclohexane-1-carboxamide) (PIN)
read 2,2′-[ethane-1,2-diylbis(azanediyl)]di(cyclohexane-1-carboxamide) (PIN) [remove space in name]
Page 850, P-66.1.1.4.2, examples 1 and 4.
Remove one as it is a duplicate of the other
Page 851, P-66.1.1.4.3, example 4.
For (2) 3-(methanesulfonylamino)propanoic acid (PIN)
read (2) 3-[(methanesulfonyl)amino]propanoic acid (PIN)
Page 851, P-66.1.1.4.3, example 5.
For (2) [(cyclohexylmethyl)amino]acetic acid
read (2) {[(cyclohexylmethyl)sulfonyl]amino}acetic acid
Page 851, P-66.1.1.4.3, example 6.
For (1) N-cyclopropyl-N-methanesulfonylglycine
read (1) N-cyclopropyl-N-(methanesulfonyl)glycine
for (2) [N-(cyclopropylmethyl)sulfonamido]acetic acid
read (2) [cyclopropyl(methanesulfonyl)amino]acetic acid
Page 853, P-66.1.1.4.5.1, example. [corrected 13 March 2019]
For 3-{amino(oxo)acetyl]imino}propanoic acid.
read 3-{[amino(oxo)acetyl]imino}propanoic acid
Page 853, P-66.1.1.4.5.2, example.
Add N1,N2-bis(cyanomethyl)oxamide (PIN)
for 2,2′-[oxalylbis(azanediyl)]diacetonitrile (PIN)
read (not 2,2′-[oxalylbis(azanediyl)]diacetonitrile)
for 2,2′-[ethanedioylbis(azanediyl)]diacetonitrile
read (not 2,2′-[ethanedioylbis(azanediyl)]diacetonitrile)
Page 854, P-66.1.2.1, example 2. [corrected 26 September 2018]
Replace the structure with:
(CH3-CO)2N–
Page 854, P-66.1.2.1, example 5.
For N,N-di(cyclohexanecarbonyl)cyclohexane-1-carboxamide (PIN)
read N,N-di(cyclohexanecarbonyl)cyclohexanecarboxamide (PIN)
Page 855, P-66.1.3, example 2.
For (1,4-dihydroquinolin-1(2H)-yl)propan-1-one (PIN)
read 1-(3,4-dihydroquinolin-1(2H)-yl)propan-1-one (PIN)
for (not 1-propionyl-1,2,3,4-tetrahydroquinoline)
read 1-propionyl-1,2,3,4-tetrahydroquinoline
Page 857, P-66.1.4.2, example 1.
For N-ethanethioylethanethioamide (PIN)
read N-(ethanethioyl)ethanethioamide (PIN)
for N-thioacetylthioacetamide
read N-(thioacetyl)thioacetamide
Page 857, P-66.1.4.2, example 2.
For N-cyclohexyl-N-ethanethioylethanethioamide
read N-cyclohexyl-N-(ethanethioyl)ethanethioamide (PIN)
for N-cyclohexyl-N-thioacetylthioacetamide
read N-cyclohexyl-N-(thioacetyl)thioacetamide
Page 857, P-66.1.4.2, example 3.
For N-propanethioylacetamide (PIN)
read N-(propanethioyl)acetamide (PIN)
Page 857, P-66.1.4.3, example.
For 1-(pyrrolidin-1-yl)ethan-1-thione (PIN)
read 1-(pyrrolidin-1-yl)ethane-1-thione (PIN)
for not 1-ethanethioylpyrrolidine
read not 1-(ethanethioyl)pyrrolidine
Page 858, P-66.1.4.4, example 1 on this page.
For 4-ethanethioamidobenzamide (PIN)
read 4-(ethanethioamido)benzamide (PIN)
for 4-(ethanethioylamino)benzamide
read 4-[(ethanethioyl)amino]benzamide
Page 858, P-66.1.4.4, example 2 on this page.
For (methanesulfinothioylamino)acetic acid (PIN)
read [(methanesulfinothioyl)amino]acetic acid (PIN)
Page 858, P-66.1.4.4, last example.
For [aminos(sulfanylidene)ethanethioyl]benzoic acid (PIN)
read 2-[amino(sulfanylidene)ethanethioyl]benzoic acid (PIN)
Page 859, P-66.1.5.2.1, example 1.
For 1λ6-2H-naphtho[1,8-cd][1,2]thiazole-1,1-dione (PIN)
read 1λ6-naphtho[1,8-cd][1,2]thiazole-1,1(2H)-dione (PIN)
Page 860, P-66.1.6.1.1.1, line 1. [corrected 26 January 2022]
For ... H2N-CO-NH2 has the retained named ‘urea’, which...
read ... H2N-CO-NH2 has the retained name ‘urea’, which...
Page 861, P-66.1.6.1.1.2, example 1. [corrected 24 October 2018]
Replace the structure with:

Page 862, P-66.1.6.1.1.3, example 4 on this page.
For N-carbamoylbenzene-1-sulfonamide (PIN)
read N-carbamoylbenzenesulfonamide (PIN)
for N-(aminocarbonyl)benzene-1-sulfonamide
read N-(aminocarbonyl)benzenesulfonamide
Page 863, P-66.1.6.1.1.5, example 2. [corrected 13 March 2019]
For 1-[3-(formylamino)propyl]urea
read N-[3-(formylamino)propyl]urea
Page 864, P-66.1.6.1.2.2, line 1.
For ...derived from isorea are named...
read ...derived from isourea are named...
Page 864, P-66.1.6.1.3.1, structures 1 and 2. [modified 7 August 2019]
For carbonothioic diamide (PIN)
read carbonothioic diamide
for thiourea
read thiourea (PIN)
replace structures with:
Page 864, P-66.1.6.1.3.1, example.
Replace the structure with:
Page 865, P-66.1.6.1.3.2, example 2 (third structure on page).
For ethyl N-methylcarbamimidothioate (PIN)
read ethyl N′-methylcarbamimidothioate (PIN)
Page 865, P-66.1.6.1.3.2, example 4.
For (not S-ethyl N-methylisothiourea)
read (not S-ethyl-N-methylisothiourea)
Page 865, P-66.1.6.1.3.3, example bottom of page.
For 3-[amino(sulfanylidene)methyl]aminopropanoic acid
read 3-{[amino(sulfanylidene)methyl]amino}propanoic acid
Page 866, P-66.1.6.1.4, example 3. [corrected 13 March 2019]
For N-methyl-2,4-diimido-3-thiotricarbonic diamide (PIN)
read N1-methyl-2,4-diimido-3-thiotricarbonic diamide (PIN)
Page 867, P-66.1.6.1.4, example 1 on this page.
For 3,5,7-trioxo-2,4,6,8-tetraazanonanediamide (PIN)
read 3,5,7-trioxo-2,4,6,8-tetraazanonane-1,9-diamide (PIN)
Page 867, P-66.1.6.3, example 1. [corrected 23 February 2022)
For N-(propan-2-yl)dicarbonic diamide (PIN)
read N1-(propan-2-yl)dicarbonic diamide (PIN)
Page 868, P-66.1.7, example 2. [modified 23 December 2020]
For N-[({[(acetamidomethyl)nitroamino]methyl}nitroamino)methyl]prop-2-enamide (PIN)
read N-{[{[(acetamidomethyl)(nitro)amino]methyl}(nitro)amino]methyl}prop-2-enamide
for N-{[({[(acetylamino)methyl]nitroamino}methyl)nitroamino]methyl}prop-2-enamide
read N-{[({[(acetylamino)methyl](nitro)amino}methyl)(nitro)amino]methyl}prop-2-enamide
add N-[({[(acetamidomethyl)nitramido]methyl}nitramido)methyl]prop-2-enamide (PIN)
Page 869, P-66.2.1, example 4.
For hexahydro-2H-isoindole-1,3-dione (PIN)
read hexahydro-2H-isoindole-1,3-dione
add hexahydro-1H-isoindole-1,3(2H)-dione (PIN)
Page 869, P-66.2.1, example 5.
For 2-phenyl-2H-isoindole-1,3-dione (PIN)
read 2-phenyl-2H-isoindole-1,3-dione
add 2-phenyl-1H-isoindole-1,3(2H)-dione (PIN)
Page 870, P-66.2.2, example 1 on this page. [corrected 19 September 2018]
For 7-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)naphthalen-1-carboxylic acid (PIN)
read 7-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)naphthalene-1-carboxylic acid (PIN)
Page 871, P-66.3.1.1, box, line 4. [corrected 26 September 2018]
For ...CH3-CH3-CH2-CH2-CO-NH-NH2, not pentanohydrazide
read ...CH3-CH2-CH2-CH2-CO-NH-NH2, not pentanohydrazide.
Page 872, P-66.3.1.1, example on this page.
Replace structure with:
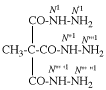
Page 875, P-66.3.2.2, example 1. [corrected 24 October 2018]
Replace the structure with:
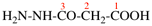
Page 875, P-66.3.2.3 (1), line 3. [corrected 13 March 2019]
For ...and ‘benzenecarbohydrazido’; the...
read ...and ‘benzohydrazido’; the...
Page 875, P-66.3.2.3, example 1. [corrected 7 August 2019]
For (1) 4-(acetohydrazido)benzoic acid (PIN)
read (1) 4-acetohydrazidobenzoic acid (PIN)
Page 877, P-66.3.3.2, example 2. [modified 11 March 2020]
For ...and '1,4,6' is lower than '1,4,7')
read ...and 'N1,N′4,6' is lower than 'N′1,N4,7')
Page 878, P-66.3.4, example 2.
For not benzenecarbothioylhydrazine);...
read not (benzenecarbothioyl)hydrazine;...
Page 879, P-66.3.5.2, example 1. [corrected 7 November 2018]
For (hydrazinecarbonyl)oxy}formohydrazide
read [(hydrazinecarbonyl)oxy]formohydrazide
Page 879, P-66.3.5.2, example 2. [corrected 7 November 2018]
For [(hydrazinecarbonyl)amino)formohydrazide
read [(hydrazinecarbonyl)amino]formohydrazide
Page 879, P-66.3.5.3, example 2.
For 4-{[(hydrazinecarbonyl)oxy)]formohydrazido}butanoic acid (PIN)
read 4-{[(hydrazinecarbonyl)oxy]formohydrazido}butanoic acid (PIN)
Page 879, P-66.3.5.3, example 3.
For hydrazinecarbohydrazidoacetic acid (PIN)
read (hydrazinecarbohydrazido)acetic acid (PIN)
for {[2-(hydrazinylcarbonyl)hydrazine-1-yl}acetic acid
read [2-(hydrazinylcarbonyl)hydrazin-1-yl]acetic acid
Page 879, P-66.3.5.3, example 4.
Delete example as it is the same as example 2
Page 880, P-66.4.1, structure 1.
Replace structure with:
Page 881, P-66.4.1.1, example 1. [corrected 13 March 2019]
Replace the structure with:
Page 883, P-66.4.1.1, example on this page.
For N′′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN)
read N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN)
replace structure with:
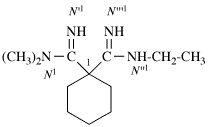
Page 883, P-66.4.1.2.1.2, example 2. [corrected 24 October 2018]
Replace the structure with:
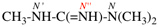
Page 884, P-66.4.1.2.1.3, example 1.
For carbaminidamido
read carbamimidamido
Page 884, P-66.4.1.2.1.3, example 5. [corrected 13 March 2019]
For carbamimidoylurea (PIN)
read N-carbamimidoylurea (PIN)
Page 884, P-66.4.1.2.1.3, example 6. [corrected 24 October 2018]
Replace the structure with:
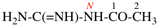
Page 885, P-66.4.1.2.2, structure 2. [corrected 24 October 2018]
Replace the structure with:
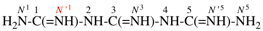
Page 885, P-66.4.1.2.2, example 3.
For N,N-diphenyl-N′′-ethylimidodicarbonimidic diamide (PIN)
read N′1-ethyl-N1,N1-diphenylimidodicarbonimidic diamide (PIN)
Replace structure with:
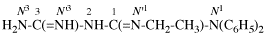
Page 885, P-66.4.1.2.2, example 4.
For 3,5,7-triimino-2,4,6,8-tetraazanonanedi(imidamide) (PIN)
read 3,5,7-triimino-2,4,6,8-tetraazanonane-1,9-diimidamide (PIN)
Page 888, P-66.4.1.4.1, example 1 on this page.
For N′-methyl-N,N-diphenylbenzene-1-carboximidamide (PIN)
read N′-methyl-N,N-diphenylbenzenecarboximidamide (PIN)
Page 888, P-66.4.1.4.1, example 2 on this page.
For N′-ethyl-N -methylbenzenecarboximidamide (PIN)
read N′-ethyl-N-methylbenzenecarboximidamide (PIN)
for (not N′-ethyl-N -methylbenzene-1-carboxamidine)
read (not N′-ethyl-N-methylbenzenecarboxamidine). [Deleted space from names]
Page 888, P-66.4.1.4.1, example 3 on this page.
For N-phenylbenzene-1-carboximidamide (PIN)
read N-phenylbenzenecarboximidamide (PIN)
for (not N-phenylbenzene-1-carboxamidine;
read (not N-phenylbenzenecarboxamidine;
Page 889, P-66.4.1.4.2, example 1.
Replace the structure with:

Page 889, P-66.4.1.5, example 1. [modified 13 March 2019]
For [not N1-ethyl-N2-methyldisulfanedicarboximidamide; not N-ethyl-N′′-methyl(dithioperoxy)dicarbonimidic diamide (numbering shown); ...
read [not N1-ethyl-N2-methyldisulfanedicarboximidamide (numbering shown); not N-ethyl-N′′-methyl(dithioperoxy)dicarbonimidic diamide; ...
for ...; not N1-ethyl-N2-methyl-α,α′-dithiobisformamidine...
read ...; not N1-ethyl-N3-methyl-α,α′-dithiobisformamidine...
Page 889, P-66.4.1.4.2, example 2.
For N1-ethyl-N′′1,N′′1,N3,N3-tetramethylcyclohexane-1,1,3-tricarboximidamide (PIN)
read N′′1-ethyl-N1,N1,N3,N3-tetramethylcyclohexane-1,1,3-tricarboximidamide (PIN)
Replace structure with:
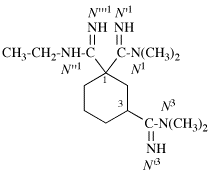
Page 891, P-66.4.2.1, example 7 on this page.
For 2-(hydrazinyl)-2-iminoethane-1-hydrazonamide (PIN)
For 2-hydrazinyl-2-iminoethanehydrazonamide (PIN)
Page 891, P-66.4.2.1, example 8 on this page.
For 4-[C-(2-nitrosohydrazine-1-carbonimidoyl)]tetraaz-3-ene-1-carboximidamide (PIN)
read 4-(2-nitrosohydrazine-1-carboximidoyl)tetraaz-3-ene-1-carboximidamide (PIN)
Page 892, P-66.4.2.1, example 1 on this page.
For N′1,N′1-diethyl-N′′′ ′1, N′′′ ′1-dimethyllcyclohexane-1,1-dicarboximidohydrazide (PIN)
read N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1-dimethylcyclohexane-1,1-dicarboximidohydrazide (PIN) [delete a space from name]
Page 893, P-66.4.2.2, example 1 on this page.
For (hydrazinecarboximidoyloxy)methanehydrazonamide (PIN)
read [(hydrazinecarboximidoyl)oxy]methanehydrazonamide (PIN)
for (carbamohydrazonoyloxy)methanimidohydrazide
read [(carbamohydrazonoyl)oxy]methanimidohydrazide
for (hydrazinecarboximidoyloxy)formohydrazonamide
read [(hydrazinecarboximidoyl)oxy]formohydrazonamide
Page 893, P-66.4.2.3.1, example 1.
For 3-imino-3-hydrazinylpropanoic acid (PIN)
read 3-hydrazinyl-3-iminopropanoic acid (PIN)
for hydrazinecarboximidoylacetic acid
read (hydrazinecarboximidoyl)acetic acid
Page 893, P-66.4.2.3.1, example 2.
For 3-hydrazinecarboximidoylbenzoic acid (PIN)
read 3-(hydrazinecarboximidoyl)benzoic acid (PIN)
Page 894, P-66.4.2.3.3, lines 2/3. [corrected 1 July 2020]
For ...and ‘(hydrazinylidenemethyl)amino’ and ‘methanehydrazonoylamino’. The preferred...
read ...and ‘(hydrazinylidenemethyl)amino’ and ‘(methanehydrazonoyl)amino’. The preferred...
Page 894, P-66.4.2.3.3, example 2.
For 3-methanehydrazonamidopropanenitrile (PIN)
read N-(2-cyanoethyl)methanehydrazonamide (PIN)
for 3-(methanehydrazonoylamino)propanenitrile
read (not 3-[(methanehydrazonoyl)amino]propanenitrile;
for 3-[(hydrazinylidenemethyl)amino]propanenitrile
read nor 3-[(hydrazinylidenemethyl)amino]propanenitrile)
Page 894, P-66.4.2.3.4, lines 1-2. [corrected 13 March 2019]
For ...the group H2N-CH=N-NH– is (aminomethylidene)hydrazin-1-yl’.
read ...the group H2N-CH=N-NH– is ‘2-(aminomethylidene)hydrazin-1-yl’.
Page 894, P-66.4.2.3.4, line 3. [corrected 13 March 2019]
For ...and ‘2-methanimidoylhydrazin-1-yl’ and...
read ...and ‘2-(methanimidoyl)hydrazin-1-yl’ and...
Page 894, P-66.4.2.3.4, line 5. [corrected 1 July 2020]
For ...(preferred prefix) and ‘methanehydrazonoylamino’.
read ...(preferred prefix) and ‘(methanehydrazonoyl)amino’.
Page 894, P-66.4.2.3.4, example. [modified 13 March 2019]
For 3-[(aminomethylidene)hydrazinyl]propanoic acid (PIN)
read 3-[2-(aminomethylidene)hydrazin-1-yl]propanoic acid (PIN)
replace the structure with:
Page 894, P-66.4.2.3.5, example 1.
For 3-ethanehydrazonamidopropanoic acid (PIN)
read 3-(ethanehydrazonamido)propanoic acid (PIN)
for
3-(ethanehydrazonoylamino)propanoic acid
read 3-[(ethanehydrazonoyl)amino]propanoic acid
Page 895, P-66.4.2.3.5, example on this page.
For 4-benzenesulfinohydrazonamidobenzoic acid (PIN)
read 4-(benzenesulfinohydrazonamido)benzoic acid (PIN)
for 4-(benzenesulfinohydrazonoylamino)benzoic acid
read 4-[(benzenesulfinohydrazonoyl)amino]benzoic acid
Page 895, P-66.4.2.3.6, example 1.
For 3-methanimidohydrazidopropanoic acid (PIN)
read 3-(methanimidohydrazido)propanoic acid (PIN)
for 3-(2-methanimidoylhydrazin-1-yl)propanoic acid
read 3-[2-(methanimidoyl)hydrazin-1-yl]propanoic acid
Page 895, P-66.4.2.3.6, example 2. [corrected 10 October 2018]
For methyl ethanimidohydrazidoacetate (PIN)
read methyl (ethanimidohydrazido)acetate (PIN)
for methyl (2-ethanimidoylhydrazin-1-yl)acetate
read methyl [2-(ethanimidoyl)hydrazin-1-yl]acetate
replace the structure with:
CH3-C(=NH)-NH-NH-CH2-CO-O-CH3
Page 895, P-66.4.2.3.6, example 3.
For 4-[2-benzenecarboximidoyl)hydrazin-1-yl]benzoic acid
read 4-[2-(benzenecarboximidoyl)hydrazin-1-yl]benzoic acid
Page 896, P-66.4.3.1, example 5. [corrected 26 September 2018]
Replace the structure with:
Page 897, P-66.4.3.1, example 1 on this page.
For 1, N′ 2, N2, N6-tetramethylnaphthalene-2,6-dicarbohydrazonohydrazide (PIN)
read N′2,N′2,N6,1-tetramethylnaphthalene-2,6-dicarbohydrazonohydrazide (PIN) [delete spaces from name]
Page 897, P-66.4.3.1, example 3 on this page.
For
N′1, N′1-diethyl-N′′′ ′1,N′′′ ′1,N3-trimethylcyclohexane-1-1-3-tricarbohydrazonohydrazide (PIN)
read N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1,N3-trimethylcyclohexane-1,1,3-tricarbohydrazonohydrazide (PIN) [remove a space from name]
Page 898, P-66.4.3.3, example 1. [modified 3 March 2021]
For carbonohydrazonic dihydrazide (P-65.2.1.5)
read carbonohydrazonic dihydrazide (P-65.2.1.3)
Page 898, P-66.4.3.4.1, example 1.
For 3-(hydrazin-1-yl)-3-hydrazinylidenepropanoic acid (PIN)
read 3-hydrazinyl-3-hydrazinylidenepropanoic acid (PIN)
for [C-(hydrazinylcarbonohydrazonoyl)]acetic acid
read (C-hydrazinylcarbonohydrazonoyl)acetic acid
Page 898, P-66.4.3.4.1, example 2.
For 3-[ C-(hydrazinylcarbonohydrazonoyl)]benzoic acid
read 3-(C-hydrazinylcarbonohydrazonoyl)benzoic acid [delete space in name]
for 3-[hydrazinyl(hydrazanylidene)methyl]benzoic acid
read 3-[hydrazinyl(hydrazinylidene)methyl]benzoic acid
Page 898, P-66.4.3.4.1, example 3.
For 4-[(carbamimidoyloxy)methanimidohydrazido]benzoic acid (PIN)
read 4-[C-(carbamimidoyloxy)methanimidohydrazido]benzoic acid (PIN)
for 4-{2-[(carbamimidoyloxy)methanimidoyl]hydrazin-1-yl}benzoic acid
read 4-{2-[C-(carbamimidoyloxy)methanimidoyl]hydrazin-1-yl}benzoic acid
Page 899, P-66.4.3.4.2, example. [modified 1 July 2020]
Delete (2-methanehydrazonoylhydrazin-1-yl)acetic acid (PIN)
for [(hydrazinylmethylidene)hydrazinyl]acetic acid
read [(hydrazinylmethylidene)hydrazinyl]acetic acid (PIN)
Page 900, P-66.5.0, lines 6-8. [corrected 26 January 2022]
For ...compound is a preferred IUPAC name. When unsubstituted, the preferred IUPAC name is hydrogen cyanide.
read ...compound is a preferred IUPAC name.
Page 900, P-66.5.0, line 6.
For ...formonitrile in the preferred IUPAC name...
read ...formonitrile is the preferred IUPAC name...
Page 902, P-66.5.1.2.1, example 3, second line.
For benzonitrile (PIN)
read benzenecarbonitrile
Page 904, P-66.5.2, example 1. [corrected 5 July 2018]
Replace structure with:
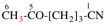
Page 904, P-66.5.2 example 6.
For (not 3,3′-iminodipropionitrile;
read (not 3,3′-azanediyldipropionitrile;
Page 905, P-66.5.3.1, example 1 and 2.
move (not oxomalononitrile; no substitution allowed on malononitrile) from example 2 to example 1 (carbonyl dicyanide)
Page 905, P-66.5.3.1, last example.
For dicarbonic dicyanide (PIN)
Add (see P-65.5.3.2)
Page 906, P-66.5.4.2, example.
For methyl 4-[(oxo-λ5-azanylidyne)methyl]benzoate (PIN)
read
4-(methoxycarbonyl)benzonitrile oxide (PIN)
add (not methyl 4-[(oxo-λ5-azanylidyne)methyl]benzoate)
replace structure with:
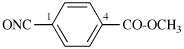
Page 906, P-66.5.4.2, new example.
Add sodium 4-[(oxo-λ5-azanylidyne)methyl]benzoate (PIN)
(no longer sodium 4-isofulminatobenzoate)
add structure:
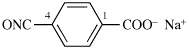
Page 907, P-66.6.1.1.2, example.
Replace structure with:
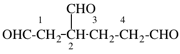
Page 908, P-66.6.1.2.1, example 3.
For benzenecarboxaldehyde
read benzenecarbaldehyde
Page 908, P-66.6.1.2.1, example 4. [corrected 7 August 2019]
Delete this entry
Page 909, P-66.6.1.3, example 1 on his page.
Replace structures with:
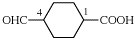
Page 909, P-66.6.1.3, example 2 on this page.
For 7-(2-formylcyclopentyl)heptanaldehyde
read 7-(2-formylcyclopentyl)heptanal
Page 909, P-66.6.2, example 1. [corrected 26 January 2022]
Delete oxydiformaldehyde
Page 909, P-66.6.2, example 2. [modified 26 January 2022]
Delete [carbonylbis(oxy)diformaldehyde]
Page 910, P-66.6.3, example 5.
For 4-methanethioylbenzoic acid (PIN)
read 4-(methanethioyl)benzoic acid (PIN)
Page 913, P-66.6.5.1.2, example on this page. [corrected 30 March 2022]
For [2-(1,3-dioxolan-2-yl)ethyl]trimethylsilane (PIN)
read [2-(1,3-dioxolan-2-yl)ethyl]tri(methyl)silane (PIN)
Page 913, P-66.6.5.3, example 1.
For pentanal diethyl dithioacetal
read pentanal dimethyl dithioacetal
Page 917, P-67.1.1.1, line 1 on this page.
For N(O)(OH)3 azoric acid (preselected name)
read N(O)(OH)3 nitroric acid (preselected name)
Page 917, P-67.1.1.1, line 12 on ths page.
For hypoflurous acid
read hypofluorous acid
Page 919, P-67.1.1.2, example 1 on this page.
For naphthalene-2,6-diylbis(phosphonous acid) (PIN)
read (naphthalene-2,6-diyl)bis(phosphonous acid) (PIN)
Page 919, P-67.1.2.1, example 4.
For N(O)(OH)3 azoric acid (hypothetical)
read N(O)(OH)3 nitroric acid (hypothetical)
Page 919, P-67.1.2.1, example 5. [corrected 25 August 2016]
For N(OH)
read N(OH)3
Page 921, P-67.1.2.3.1, example (3). [corrected 25 August 2016]
For ...telluroperoxo
read ...ditelluroperoxo
Page 922, P-67.1.2.3.2. [corrected 1 November 2023]
For P-67.1.2.3.2 Infixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.2 Infixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2) having the same rank within the class.
Page 922, P-67.1.2.3.4. [corrected 1 November 2023]
For P-67.1.2.3.4 Prefixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.4 Prefixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2)
having the same rank within the class.
Page 923, P-67.1.2.4.1.1, example 5. [corrected 9 April 2025]
For N,N-dimethylphosphoramidic acid (PIN)
read dimethylphosphoramidic acid (PIN)
Page 923, P-67.1.2.4.1.1, example 9.
For CH3)2N-P(O)(NCS)(SH)
read (CH3)2N-P(O)(NCS)(SH)
Page 923, P-67.1.2.4.1.1, example 9. (corrected 4 December 2019)
For N,N-dimethylphosphoramido(isothiocyanatido)thioic S-acid (PIN)
read N,N-dimethylphosphoramid(isothiocyanatido)thioic S-acid (PIN)
Page 924, P-67.1.2.4.1.1, example 1 on this page. [modified 25 November 2020]
For N,N-dimethyl-N′ -phenylphosphoramidimido(thiocyanatidic) acid (PIN)
read N,N-dimethyl-N′-phenylphosphoramidimido(thiocyanatidic) acid (PIN) [delete space from name]
Page 924, P-67.1.2.4.1.1, example 2 on this page,
For phenylphosphonothious S-acid (PIN)
read phenylphosphonothious acid (PIN)
Page 924, P-67.1.2.4.1.1, example 7 on this page. [corrected 25 August 2016]
For P(=NH)(NH-NH2)(OH)
read P(=NH)(NH-NH2)(OH)2
Page 924, P-67.1.2.4.1.1, example 12 on this page. [modified 20 March 2019]
For diphenylarsorothious acid (PIN)
read diphenylarsinothious acid (PIN)
Page 924, P-67.1.2.4.1.1, example 17 on this page. [modified 20 March 2019]
For sulfuro(isothiocyanatidic) acid
read sulfur(isothiocyanatidic) acid
For HO-SO-NCS
read HO-SO2-NCS
Page 925, P-67.1.2.4.1.3, example 1. [corrected 25 August 2016, modified 10 October 2018]
For methylphophonocyanatidic acid (PIN)
read methylphosphonocyanatidic acid (PIN)
for CH3-P(OCN)OH
read CH3-P(O)(OCN)OH
Page 925, P-67.1.2.4.2, line 1.
For ...phosphinous, phosponic and phosphinic...
read ...phosphinous, phosphonic and phosphinic...
Page 925, P-67.1.2.4.2, lines 4-6.
For ...and not chlorophenylphosphinic acid; (C5H10N)-P(O)Cl(OH) is piperidin-1-ylphosphonochloridic acid and not chloropiperidin-1-ylphosphinic acid; and...
read ...and not chloro(phenyl)phosphinic acid; (C5H10N)-P(O)Cl(OH) is (piperidin-1-yl)phosphonochloridic acid and not chloro(piperidin-1-yl)phosphinic acid; and...
Page 926, P-67.1.2.5.1, example 11. Correction deleted [11 November 2020]
Page 927, P-67.1.2.5.1, example 1 on this page. [corrected 26 September 2018]
For S(=NH-CH3)Cl2
read S(=N-CH3)Cl2
Page 927, P-67.1.2.5.2, example 1. [corrected 30 March 2022]
For bromo(chloro)phenylborane (PIN)
read bromo(chloro)(phenyl)borane (PIN)
Page 927, P-67.1.2.5.2, example 2.
Replace structure with:
CH3-SiCl3 [delete space in structure]
Page 927, P-67.1.2.5.2, example 3, (with last two names under example 2).
Add tetrachlorosilane (preselected name)
add structure: SiCl4
Page 927, P-67.1.2.6.1 (b), box.
For 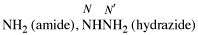
read 
Page 928, P-67.1.2.6.1, example 5.
For N,N-diethyl-N′,N′-di methyl-Sb-phenylstibonothioic diamide (PIN)
read N,N-diethyl-N′,N′-dimethyl-Sb-phenylstibonothioic diamide (PIN) [Deleted space from name]
Page 929, P-67.1.2.6.1, example 1 on this page. [corrected 25 August 2016]
For N,N,N,N′-tetramethyl-N-phenylsulfurimidic diamide (PIN)
read N,N,N′,N′-tetramethyl-N′′-phenylsulfurimidic diamide (PIN)
Page 929, P-67.1.2.6.2, example 3.
For 1,1′,1′′-boranetriyltris(hydrazine)
read 1,1′,1′′-boranetriyltrihydrazine
Page 929, P-67.1.2.6.2, example 5.
For 1,1′,1′′,1′′′-silanetetrayltetrakis(hydrazine)
read 1,1′,1′′,1′′′-silanetetrayltetrahydrazine
Page 929, P-67.1.2.6.2, last example.
Replace structure with:
Si(NH-NH2)4
Page 930, P-67.1.2.6.3, example 1. [corrected 24 March 2021]
For N,N-dipropylnitrous amide (PIN)
read dipropylnitrous amide (PIN)
Page 930, P-67.1.2.6.3, example 2. [corrected 24 March 2021]
For N-butyl-N-ethylnitrous amide (PIN)
read butyl(ethyl)nitrous amide (PIN)
Page 930, P-67.1.2.6.3, example 3. [corrected 24 March 2021]
For N-(chloromethyl)-N-methylnitramide (PIN)
read (chloromethyl)(methyl)nitramide (PIN)
Page 930, P-67.1.2.6.3, example 4. [corrected 24 March 2021]
For N-methyl-N-nitronitramide (PIN)
read methyl(nitro)nitramide (PIN)
Page 932, P-67.1.3.2, example 1 on this page.
For P(O)(CH3)3
read P(O)(O-CH3)3
Page 932, P-67.1.3.2, example 4 on this page. [corrected 16 August 2016]
For O-methyl phenylarsinothioate
read O-methyl phenylarsinothioate (PIN)
Page 932, P-67.1.3.2, example 15 on this page. [corrected 7 November 2018]
For Sb(O)(F2)(S-CH3)
read Sb(O)(F)2(S-CH3)
Page 933, P-67.1.3.2, example 1 on this page.
Replace the structure with:
Si(S-CH3)3(O-CH2-CH3)
Page 933, P-67.1.3.2, example 2 on this page.
For S,S′-diethyl O,O′-dimethyl P,P′-ethane-1,2-diylbis(phosphonothioate)(PIN)
read S,S′-diethyl O,O′-dimethyl P,P′-(ethane-1,2-diyl)bis(phosphonothioate) (PIN)
Page 935, P-67.1.4.1.1.2, examples 5-7.
For –NH(O)< azonoyl
read NH(O)< azonoyl
for –NH2(O)< azinoyl
read NH2(O)– azinoyl
for –PH(O)< phosphonoyl
read PH(O)< phosphonoyl
Page 936, P-67.1.4.1.1.2, examples 1-5 on this page.
For –PH2(O)< phosphinoyl
read PH2(O)– phosphinoyl
for –AsH(O)< arsonoyl
read AsH(O)< arsonoyl
for –AsH2(O)< arsinoyl
read AsH2(O)– arsinoyl
for –SbH(O)< stibonoyl
read SbH(O)< stibonoyl
for –SbH2(O)< stibinoyl
read SbH2(O)– stibinoyl
Page 937, P-67.1.4.1.1.4, example 3, right version. [corrected 20 March 2019]
For hydrazonostiboroyl
read hydrazonostiboryl
Page 938, P-67.1.4.1.1.4, example 5 left on this page. (corrected 27 November 2019)
For dimethylphosphoramidic acid (PIN)
read N,N-dimethylphosphoramidic acid (PIN)
Page 938, P-67.1.4.1.1.4, example 5 right on this page. (modified 27 November 2019)
For dimethylphosphoramidoyl (preferred prefix)
read N,N-dimethylphosphoramidoyl (preferred prefix)
for (diimethylamido)phosphoryl
read (dimethylamido)phosphoryl
Page 941, P-67.1.4.1.3, example 1.
For 2-(phosphonooxy)acetic acid (PIN)
read (phosphonooxy)acetic acid (PIN)
Page 941, P-67.1.4.2, example 2. [corrected 25 August 2016]
For (not borinoyl)
read (not boryl)
Page 943, P-67.1.4.3.2 example 6.
For (sulfanylideneimino)sulfonyl (preselected prefix)
read (sulfanylideneamino)sulfonyl (preselected prefix)
Page 943, P-67.1.4.3.3, line 3. [corrected 25 August 2016]
For ...appropriate substituent group ‘hydrazine-1-yl’, ‘hydrazine-1-ylidene’, etc.
read ...appropriate substituent group ‘hydrazin-1-yl’, ‘hydrazin-1-ylidene’, etc.
Page 944, P-67.1.4.4.1, example 10. (corrected 4 December 2019)
For sulfurisothiocyanatidoyl
read sulfur(isothiocyanatidoyl)
Page 945, P-67.1.4.4.2, example 4. [corrected 25 August 2016]
For [not (sulfonamidoyloxy)propanoic acid; ...
read [not 3-(sulfonamidoyloxy)propanoic acid; ...
Page 947, P-67.1.5.1, example 2 on this page. [modified 22 April 2020]
For N-[tris(phenylamino)-λ5-phosphanylidene]benzenesulfonamide (PIN)
read N-(trianilino-λ5-phosphanylidene)benzenesulfonamide (PIN)
for N-[tris(phenylamino)phosphoranylidene]benzenesulfonamide
read N-(trianilinophosphoranylidene)benzenesulfonamide
For (N,N′,N′′-triphenyl-N′′′-benzenesulfonylphosphorimidic triamide;
read [N,N′,N′′-triphenyl-N′′′-benzenesulfonylphosphorimidic triamide;
for nor N′′′-benzenesulfonylphosphorimimidic tris(phenylamide)
read nor N′′′-benzenesulfonylphosphorimimidic tris(phenylamide)]
Page 948, P-67.1.6, example 1 on this page.
For (dimethylazinoyl)acetonitrile (PIN)
read (dimethylazinoyl)acetonitrile
add cyano-N,N-dimethylmethanamine N-oxide (PIN; see P-62.5)
Page 949, P-67.2.1, example 8. [corrected 25 August 2016]
For HO)2P(O)-O-P(O)(OH)2
read (HO)2P(O)-O-P(O)(OH)2
Page 949, P-67.2.1, example 10. [corrected 25 August 2016]
For HO)2P-O-P(OH)2
read (HO)2P-O-P(OH)2
Page 949, P-67.2.1, last example on this page. [corrected 25 August 2016]
For HO)2As(O)-O-As(O)(OH)2
read (HO)2As(O)-O-As(O)(OH)2
Page 950, P-67.2.1, example 2 on this page. [corrected 25 August 2016]
For HO)2As-O-As(OH)2
read (HO)2As-O-As(OH)2
Page 950, P-67.2.1, lines 16/17.
Delete HO-SO-O-SO-OH disulfurous acid (preselected name; (see P-67.3.2)
Page 952, P-67.2.2.2, example 1 [corrected 25 August 2016]
For (not 1,3-dithiotriphosphoric 1-S , 3 - S - acid)
read (not 1,3-dithiotriphosphoric 1-S,3-S-acid) [delete spaces in name]
Page 952, P-67.2.2.2, example 4. [modified 20 March 2019]
For 1,3-dithiodiphosphonic O1,S2-acid
read 1,3-dithiodiphosphonic O1,S3-acid
for ...name diphosphoric acid...
read ...name diphosphonic acid...
Page 954, P-67.2.2.2, example 1 on this page.
For N-methyl-1-imido-2-thiodithionous S2-acid (PIN;
read N1-methyl-1-imido-2-thiodithionous S2-acid (PIN;
and replace structure with:
Page 955, P-67.2.3, example 5. [corrected 25 August 2016]
For 1-amido-2-imidodiphosphonic chloride
read 3-amido-2-imidodiphosphonic chloride
Replace the structure with:
Page 957, P-67.2.4, example 3 on this page. [corrected 25 August 2016]
For N1methyl-1,2,3-triimidodiphosphoric tetraamide (PIN)
read N1-methyl-1,2,3-triimidodiphosphoric tetraamide (PIN)
Page 958, P-67.2.4, example 2 on this page. [corrected 25 August 2016]
For heptaimidoimidotetraphosphoric hexaamide
read heptaimidotetraphosphoric hexaamide
Page 959, P-67.2.5.1.1, example 1.
For disodium N-methyl-1,2-diimidodithionite (PIN)
read disodium N1-methyl-1,2-diimidodithionite (PIN)
and replace structure with:
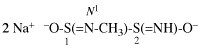
Page 959, P-67.2.5.2, example 4. [corrected 11 December 2023]
For (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 di(propan-2-yl) disulfite (PIN)
read (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 1,3-bis[(propan-2-yl)oxy]-1λ4,3λ
[not di(propan-2-yl) disulfite, see P-67.3.2]
Page 960, P-67.2.6, example 1.
For (1) 3-{[hydroxy(phosphonooxy)phosphoryl)]oxy}propanoic acid
read (1) 3-{[hydroxy(phosphonooxy)phosphoryl]oxy}propanoic acid
Page 960, P-67.2.6, example 2. [corrected 25 August 2016]
For (1) [({[(methoxysulfinyl)oxy]sulfinyl}oxy)sulfinyl]oxy}acetic acid
read (1) {[({[(methoxysulfinyl)oxy]sulfinyl}oxy)sulfinyl]oxy}acetic acid
Page 961, P-67.2.6, example on this page. [modified 20 March 2019]
For (3) 3,5-disulfanylidene-2,3λ4,4,5λ4,6-pentathianonan-9-oic acid (PIN)
read (3) 3,5-bis(sulfanylidene)-2,3λ4,4,5λ4,6-pentathianonan-9-oic acid (PIN)
for (2) 3-(5-methyl-2,4-sulfanylidene-2λ4,4λ4-pentasulfanyl)propanoic acid
read (2) 3-[5-methyl-2,4-bis(sulfanylidene)-2λ4,4λ4-pentasulfanyl]propanoic acid
Page 961, P-67.3.1, lines 9-13. [corrected 20 March 2019]
For ...acids, acid halides, azides, amides, hydrazides, cyanides, isocyanides, isocyanates (and chalcogen analogues in the order O > S > Se > Te), imides, and nitrides and the maximum number of groups representing senior classes.
read ...acids, acid halides, azides, cyanides, isocyanides, isocyanates (and chalcogen analogues in the order O > S > Se > Te), amides, hydrazides, and the maximum number of groups representing the senior class (see P-67.1.2.3.2).
Page 962, P-67.3.1, example 5 on this page. [corrected 25 August 2016]
For bis(trimethylphosphanyl)phosphinous acid (PIN)
read bis(dimethylphosphanyl)phosphinous acid (PIN)
Page 962, P-67.3.1, example 6 on this page. [corrected 25 August 2016]
For (not orthosulfurodiiodidothiocyanatous acid)
read (not orthosulfurodiiodidothiocyanatidous acid)
Page 963, P-67.3.1, example 1 on this page.
For CH3-CO-O-P(OH)2
read CH3-CO-O-P(O)(OH)2
Page 963, P-67.3.2, box last line.
For 1,3-dihydroxydisulfoxane-1,3-dione
read 1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione
Page 963, P-67.3.2, example 1.
For 1,3-dihydroxydisulfoxane-1,3-dione (preselected name)
read 1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione (preselected name)
replace structure with:
HO-SO-O-SO-OH
Page 963, P-67.3.2, example 2.
For 1,3-dimethoxydisulfoxane-1,3-dione (PIN)
read 1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-dione (PIN)
replace structure with:
CH3-O-SO-O-SO-O-CH3
Page 963, P-67.3.2, example 3.
For 1,3-diimino-1,3-dimethoxydisulfoxane-1,3-dione (PIN)
read 1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-diimine (PIN)
Page 964, P-68.0, line 17. [corrected 28 October 2016]
For ... is named by functional class nomenclature as an ester, i.e., methyl phenylphosphinate, but the...
read ... is named by functional class nomenclature as an ester, i.e., methyl phenylphosphinite, but the...
Page 968, P-68.1.1.3.2, example 1.
For 1,3,6,2-trioxaaluminocane
read 1,3,6,2-trioxaluminocane
Page 968, P-68.1.1.3.2, example 8 on this page. [modified 11 November 2020]
For boroxin
read boroxin (see D-7.54, ref. 1)
for (not 1,3,5-trioxa-2,4,6-cyclotriboroxane)
read (not 1,3,5-trioxa-2,4,6-triboracyclohexane)
Page 969, P-68.1.1.3.2.2 (to be P-68.1.1.3.3), example 2. [corrected 16 August 2016]
For 2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane
read 2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane (PIN)
Page 970, P-68.1.2, example 10.
For H2Ga–
read H2Ga– gallanyl
Page 970, P-68.1.2, example 15. [corrected 20 March 2019]
For [1,3,2]diazaborino[1,2-a][1,3,2]diazaborin-2-yl
read [1,3,2]diazaborinino[1,2-a][1,3,2]diazaborinin-2-yl
Page 971, P-68.1.3, example 2.
For 1-methyldecahydro-1-benzoaluminine
read 1-methyldecahydro-1-benzaluminine
Page 972, P-68.1.4.1, example 4 on this page. [modified 20 March 2019]
For N,N′-bis(diphenylboranyl)ethane-1,2-diamine) (PIN)
read N1,N2-bis(diphenylboranyl)ethane-1,2-diamine (PIN)
for (not N,N-ethane-1,2-diylbis(diphenylborinic amide);
read (not N,N′-(ethane-1,2-diyl)bis(diphenylborinic amide);
for not N,N’-ethane-1,2-diylbis(diphenylboranamine);
read not N,N′-(ethane-1,2-diyl)bis(diphenylboranamine);
replace the structure with:
Page 973, P-68.1.5, suheadings.
For P-69.1.5.1 Suffix nomenclature
read P-68.1.5.1 Suffix nomenclature
for P-69.1.5.2 Prefix nomenclature
read P-68.1.5.2 Prefix nomenclature
Page 973, P-68.1.5.1, example 3. [corrected 11 March 2020]
For hydroxydimethylthallane
read hydroxydi(methyl)thallane
Page 974, P-68.1.5.2.1, example 6.
For [(ethane-1,2-diylbis(oxymethylene))bis(dimethylborane)
read [ethane-1,2-diylbis(oxymethylene)]bis(dimethylborane)
Page 974, P-68.5.2.1, example 7. [modified 11 March 2020]
Delete 3,10-diethyl-4,9-dioxa-3,10-diaza-5,8-diboradodecane (PIN)
for O,O′-[(ethane-1,2-diylbis(oxymethylene))bis(dimethylborane)]bis(N,N-diethylhydroxylamine)
read O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
add N,N′-[ethane-1,2-diylbis(boranediyloxy)]bis(N-ethylethanamine) (PIN)
Page 977, P-68.1.5.2.3, example 1 on this page.
For [not N,N′ -boranediylbis[1,1,1-trimethyl(N-trimethylsilyl)silanamine];
read [not N,N′-boranediylbis[1,1,1-trimethyl-N-(trimethylsilyl)silanamine]; [delete space in name]
Page 977, P-68.1.5.2.3, example 2 on this page.
For [chloro(2,4,6-tri-tert-butylphenyl)gallanyl]-1,1,1-trimethyl-N-(trimethylsilyl)silanamine (Si is senior to Ga)
read N-[chloro(2,4,6-tri-tert-butylphenyl)gallanyl]-1,1,1-trimethyl-N-(trimethylsilyl)silanamine (Si is senior to Ga)
Page 977, P-68.1.5.2.3, example 3 on this page.
For N,N,N′,N′-tetrakis(trimethylsilyl)-1-[(2,4,6-tri-tert-butylphenyl)indiganediamine
read 1-(2,4,6-tri-tert-butylphenyl)-N,N,N′,N′-tetrakis(trimethylsilyl)indiganediamine
replace structure with:
Page 977, P-68.1.5.2.3, last example on this page.
For (phenylgallanediyl)bis(phenylphosphane)
read P,P′-(phenylgallanediyl)bis(phenylphosphane)
Page 978, P-68.1.5.2.3, example 1 on this page.
For 4,4′,4′′-boranetriyltris(benzeneamine)
read 4,4′,4′′-boranetriyltris(benzen-1-amine)
Page 978, P-68.1.5.2.3, example 4 on this page. [corrected 30 Mach 2022]
For (difluoroboranyl)trimethylsilane (PIN)
read (difluoroboranyl)tri(methyl)silane (PIN)
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 3 on this page. [corrected 28 October 2016]
For ethaneamine(N→B2)(N→B4)pentaborane(9) (2/1)
read ethanamine(N→B2)(N→B4)pentaborane(9) (2/1)
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 4 on this page. [modified 11 September 2919]
For μ-(ethane-1,2-diamine-1κN-2κN′)bis(trihydridoboron)
read μ-(ethane-1,2-diamine-1κN1,2κN2)bis(trihydridoboron)
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 5 on this page. [corrected 20 March 2019]
For trimethylamine—(methylsulfanyl)methane—dodecaborane(10) (1/1/1)
read (trimethyl)amine—(methylsulfanyl)methane—dodecaborane(10) (1/1/1)
Page 982. P-68.1.6.2 (formerly P-68.1.6.1.1), example 1 on this page. [corrected 11 September 2919]
For (2,2′-bipyridine-κN1,κN1′)(2,2′-oxybis(benzen-1-ido-κC1)]boron(1+) perchlorate
read (2,2′-bipyridine-κ2N1,N1′)(2,2′-oxybis(benzen-1-ido-κC1)]boron(1+) perchlorate
Page 982, P-68.1.6.2 (formerly P-68.1.6.1.1), example 2 on this page. [modified 20 March 2019]
For N,N,N′,N′-tetramethylguanidine(N′′—Ga)gallane
read N,N,N′,N′-tetramethylguanidine(N′′—Ga)gallane (1/1)
delete 1,1,3,3-tetramethylguanidine(N2—Ga)gallane (1/ 1)
for trihydrido[1,1,3,3-tetramethylguanidine-κN2]gallium
read trihydrido[N,N,N′,N′-tetramethylguanidine-κN′′]gallium
Page 982, P-68.1.6.3 (formerly P-68.1.6.1.2), example 1.
For (2-amino-κN-ethan-1-olato-κO)dimethanidoboron
read (2-amino-κN-ethan-1-olato-κO)dimethanidoboron [κ not italic]
Page 982, P-68.1.6.3 (formerly P-68.1.6.1.2), example 2.
For dichlorido(2-nitro-κO-phenolato-κO)boron
read dichlorido(2-nitro-κO-phenolato-κO)boron [κ not italic]
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 3 on this page.. [modified 12 May 2021]
For (N3—B)[1H-benzimidazol-2-yl)phenyl]boronic acid
read (N3—B)[2-(1H-benzimidazol-2-yl)phenyl]boronic acid
add (N3—B)[2-(1H-1,3-benzimidazol-2-yl)phenyl]boronic acid
for [2-(1H-benzimidazol-2-yl-κN3)benzenido-κC1]dihydroxidoboron
read [2-(1H-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
add [2-(1H-1,3-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 1 on this page. [corrected 28 October 2016]
For [3,3′,3′′-nitrilo-κN-tris(propane-1-yl-κC1)]boron
read [3,3′,3′′-nitrilo-κN-tris(propan-1-yl-κC1)]boron
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 2 on this page. [corrected 28 October 2016]
For [2(O—B)]-N-[(difluoroboranyl)oxy]-N-nitrosomethanamine
read (O—B)-N-[(difluoroboranyl)oxy]-N-nitrosomethanamine
for difluorido(N-nitroso-κO-N-oxido-kO-methanamine)boron
read difluorido(N-nitroso-κO-N-oxido-κO-methanamine)boron
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 3 on this page.
For (N 3—B)[1H-benzimidazol-2-yl)phenyl]boronic acid
read (N3—B)[2-(1H-benzimidazol-2-yl)phenyl]boronic acid
for [2-(1H-benzimidazol-2-yl-κN 3)benzenido-κC1]dihydoxidoboron
read [2-(1H-benzimidazol-2-yl-κN3)benzen-l-ido-κC1]dihydoxidoboron
Page 984, P-68.1.6.3 (was P-68.1.6.1.2), example 2 on this page (example 8 of this section). [modified 9 April 2025]
For (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN:2κN′-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)bis[di(ethanido-1κ2C1,2κ2C1)gallium]
read (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN1:2κN2-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)]})bis[di(ethanido-1κ2C1,2κ2C1)gallium]
Page 985, P-68.2.1.1, example 3. [corrected 28 October 2016]
Replace the structure with:
Page 986, P-68.2.1.2, example 4.
For 2,4,6,8,10-pentathia-1,3,5,7tetrasilaadamantane
read 2,4,6,8,9,10-hexathia-1,3,5,7-tetrasilaadamantane
Page 987, P-68.2.2, example 17 on this page.
Replace diagram with:
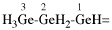
Page 988, P-68.2.3, lines 1/2. [corrected 28 October 2016]
For ...and saturation of mancude structures...
read ...and saturation of double bonds of mancude structures...
Page 989, P-68.2.4, last example.
For 1,1′,1′′,1′′′-silanetetrayltetrakis(hydrazine)
read 1,1′,1′′,1′′′-silanetetrayltetrahydrazine
Page 989, P-68.2.5, example 1.
For methylsilanetriamine (PIN)
read 1-methylsilanetriamine (PIN)
Page 989, P-68.2.5, example 2.
For N,N′ -disilyl-1-methylsilanediamine (PIN)
read 1-methyl-N,N′-disilylsilanediamine (PIN) [Deleted space from name]
Page 989, P-68.2.5, example 7. [corrected 18 September 2019]
For 4-[(bis(silanyl)methyl]hexan-2-one
read 4-[bis(silanyl)methyl]hexan-2-one
replace the structure with:
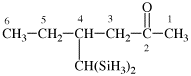
Page 989, P-68.2.5, last example on this page. [corrected 28 October 2016]
Replace the structure with:
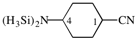
Page 990, P-68.2.5, example 1 on this page.
For dimethyl stannanediyl dibutanedioate (PIN;
read dimethyl dibutylstannanediyl dibutanedioate (PIN;
for dibutyl[(3-methoxy-3-oxopropanoyl)-3-oxy]stannane
read dibutylbis[(4-methoxy-4-oxobutanoyl)oxy]stannane
Page 990, P-68.2.5, last example.
For bis[(trimethylsilyl)methyl]stannanol (PIN)
read bis[bis(trimethylsilyl)methyl]stannanol (PIN)
for hydroxybis[(trimethylsilyl)methyl]stanane
read [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
Page 990 P-68.2.6.1, example 1.
Following P-16.5.2.6 replace the structure with:
CH3-[CH2]11-SiH2-[CH2]11-CH3
Page 991 P-68.2.6.2, example 1.
Following P-16.5.2.6 replace the structure with:
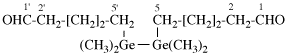
Page 991, P-68.2.6.1, example 2 on this page. [corrected 5 June 2019]
For bis(4,5-dihydrothiophen-2-yl)dimethylgermane (PIN)
read bis(4,5-dihydrothiophen-2-yl)di(methyl)germane (PIN)
Page 992, P-68.2.6.2, example 1 on this page. [modified 1 July 2020]
For methoxydimethyl[(2-(trimethylgermyl)phenyl]silane (PIN)
read methoxydi(methyl)[2-(trimethylgermyl)phenyl]silane (PIN)
Page 992, P-68.2.6.2, example 2 on this page.
For 1,4-phenylenebis(dimethylsilane) (PIN)
read (1,4-phenylene)bis(dimethylsilane) (PIN)
Page 992, P-68.2.6.2, example 3 on this page.
For thiophene-2,5-diylbis(trimethylstannane)
read (thiophene-2,5-diyl)bis(trimethylstannane)
Page 992, P-68.2.6.2, last example.
For [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane) (PIN)
read [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
add bis[bis(trimethylsilyl)methyl]stannanol (PIN)
for (Si preferred to Sn)
read [Decided by P-41 table 4.1 class 17, not class 26 or class 28]
Page 992, P-68.3, line 3.
For P-68.3.1 Nitrogen compound...
read (1) Nitrogen compound...
Page 992, P-68.3 (1) (formerly P-68.3.1), line 5. [corrected 31 July 2019]
For ...similarities, azonic HN(OH)2 and azinic acids H2N(OH), are...
read ...similarities, azonic HN(O)(OH)2 and azinic acids H2N(O)(OH), are...
Page 992, P-68.3, line 9.
For P-68.3.2 Phosphorus, arsenic,...
read (2) Phosphorus, arsenic,...
Page 992, P-68.3, line 13.
For P-68.3.3 Bismuth compounds...
read (3) Bismuth compounds...
Page 993, P-68.3.1.1.1.1, example 1.
Add N-hydroxymethanamine (PIN)
Page 993, P-68.3.1.1.1.1, example 2.
Add N-hydroxy-N-methylmethanamine (PIN)
Page 994, P-62.3.1.1.1.1, example 3 on this page. [corrected 24 July 2019]
For (not acetohydroxamic acid)
read acetohydroxamic acid
Page 994, P-68.3.1.1.1.2, example 3.
For O,O′-ethane-1,2-diylbis(hydroxylamine) (PIN)
read O,O′-(ethane-1,2-diyl)bis(hydroxylamine) (PIN)
Page 994, P-68.3.1.1.1.2, example 4. [modified 3 March 2021]
For O-benzoylhydroxylamine (PIN)
read O-benzoylhydroxylamine
for (not azanyl acetate)
read (not azanyl benzoate)
add aminooxy(phenyl)methanone (PIN)
Page 994, P-68.3.1.1.1.2, last example on this page. [corrected 28 October 2016]
For (not 1-methyldioxazane)
read (not 1-methyldiazoxane)
Page 995, P-68.3.1.1.1.3, example 1.
For N,O-dimethoxyhydroxylamine
read N,O-dimethylhydroxylamine
Page 995, P-68.3.1.1.1.3, example 3.
For O,O′-[(ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
read O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
Page 996, P-68.3.1.1.1.5, example 2.
For 2-(aminooxy)ethanamine (PIN)
read 2-(aminooxy)ethan-1-amine (PIN)
Page 997, P-68.3.1.1.2, lines 1/2 on this page. [corrected 11 December 2019]
For ...substitutively as ‘ylidene’ derivatives of hydroxylamine. Compounds...
read ...substitutively as N-hydroxy derivatives of imines. Compounds...
Page 997, P-68.3.1.1.2, box, line 2. [corrected 11 December 2019]
For ...substitutively as ‘ylidene’ derivatives of hydroxylamine rather...
read ...substitutively as N-hydroxy derivatives of imines rather...
Page 997, P-68.3.1.1.2, example 1. [corrected 24 July 2019]
For (pentan-2-ylidene)hydroxylamine (PIN)
read (pentan-2-ylidene)hydroxylamine
add N-hydroxypentan-2-imine (PIN)
Page 997, P-68.3.1.1.2, example 2. [corrected 26 September 2018]
Replace the structure with:
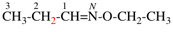
Page 997, P-68.3.1.1.2, example 3. [corrected 24 July 2019]
For butane-2,3-diylidenebis(hydroxylamine) (PIN)
read (butane-2,3-diylidene)bis(hydroxylamine)
add N2,N3-dihydroxybutane-2,3-diimine (PIN)
Page 997, P-68.3.1.1.2, example 6.
For 4-[(ethoxyimino)methyl)]benzene-1-sulfonic acid (PIN)
read 4-[(ethoxyimino)methyl]benzene-1-sulfonic acid (PIN)
Page 998, P-68.3.1.1.2, example on this page.
For pentane-2,4-diylidenebis(O-phenylhydroxylamine) (PIN)
read N2,N4-diphenoxypentane-2,4-diimine (PIN)
Page 998, P-68.3.1.1.3, example 1. [corrected 24 July 2019]
For N-(1-nitropropylidene)hydroxylamine (PIN)
read N-(1-nitropropylidene)hydroxylamine
add N-hydroxy-1-nitropropan-1-imine (PIN)
Page 998, P-68.3.1.1.3, example 2. [modified 4 September 2019]
For N-(1-nitrosoethylidene)hydroxylamine (PIN)
read N-(1-nitrosoethylidene)hydroxylamine
add N-hydroxy-1-nitrosoethan-1-imine (PIN)
Page 998, P-68.3.1.2.1, line 2.
For hetrane name
read heterane name
Page 998, P-68.3.1.2.1, box line 4.
For ... –NH-NH– ‘hydrazinediylidene’. The ...
read ... –NH-NH– ‘hydrazine-1,2-diyl’. The ...
Page 1000, P-68.3.1.2.2, example 3. [modified 18 May 2023]
For ethane-1,2-diylidenebis(phenylhydrazine) (PIN)
read 1,1′-ethanediylidenebis(2-phenylhydrazine) (PIN)
Page 1002, P-68.3.1.2.4, example 2. [corrected 28 October 2016]
For N,1-dimethylhydrazin-1-carboxamide (PIN)
read N,1-dimethylhydrazine-1-carboxamide (PIN)
Page 1002, P-68.3.1.2.4, example 5 on page.
For 3-(2-carbamoylhydrazin-1-yl)propanenitrile (PIN)
read 3-(2-carbamoylhydrazin-1-yl)propanoic acid (PIN)
replace structure with:
Page 1003, P-68.3.1.2.5, example 1. [corrected 28 October 2016]
For (hexan-3-ylidene)-N,N-diphenylhydrazinecarboxamide (PIN)
read 2-(hexan-3-ylidene)-N,N-diphenylhydrazine-1-carboxamide (PIN)
For (1-ethylbutylidene)-N,N-diphenylhydrazinecarboxamide
read 2-(1-ethylbutylidene)-N,N-diphenylhydrazine-1-carboxamide
Page 1003, P-68.3.1.2.5, last example on this page. [corrected 28 October 2016]
For N,N-diphenyl(1-phenylbutylidene)hydrazine-1-carbothioamide (PIN)
read N,N-diphenyl-2-(1-phenylbutylidene)hydrazine-1-carbothioamide (PIN)
Page 1004, P-68.3.1.2.6, line 1. [corrected 28 October 2016]
For P-68.3.1.2.6.1 The preferred...
read The preferred...
Page 1004, P-68.3.1.2.6, example 1. [corrected 28 October 2016]
Replace the structure with:
Page 1004, P-68.3.1.2.6, 2nd paragraph, lines 1/2. (corrected 27 November 2019)
For ...are called ‘2-(hydrazinecarbonyl)hydrazin-1-yl’ and ‘(hydrazinecarbonyl)hydrazinylidene’...
read ...are called ‘hydrazinecarbohydrazido’ and ‘(hydrazinecarbonyl)hydrazinylidene’...
Page 1004, P-68.3.1.2.6, 2nd paragraph, last example. (corrected 27 November 2019)
For 3-[2-(hydrazinecarbonyl)hydrazin-1-yl]propanoic acid (PIN)
read 3-[2-(hydrazinecarbonyl)hydrazin-1-yl]propanoic acid
add 3-(hydrazinecarbohydrazido)propanoic acid (PIN)
Page 1005, P-68.3.1.3.1, example 4.
For 3-(diazenyl)propanoic acid (PIN)
read 3-diazenylpropanoic acid (PIN)
Page 1007, P-68.3.1.3.2.2, method (1) example 2,
For naphthalen-2-yl(phenyl)diazene (PIN)
read (naphthalen-2-yl)(phenyl)diazene (PIN)
Page 1008, P-68.3.1.3.2.3, example 1. [modified 30 March 2022]
For (anthracen-2-yl)[(7-phenyldiazenyl)naphthalen-2-yl]diazene (PIN)
read (1) {7-[(anthracen-2-yl)diazenyl]naphthalen-2-yl}(phenyl)diazene (PIN)
for {not [7-(anthracen-2-yldiazenyl)naphthalen-2-yl]phenyldiazene
(numbering shown); alphanumerical order ‘anthracen-2-yl’ precedes ‘anthracen-2-yldiazenyl’}
read {not (anthracen-2-yl)[7-(phenyldiazenyl)naphthalen-2-yl]diazene;
each primary substituent has the same locant, i.e., none, so ‘anthracenyldiazenyl’ is alphabtically preferred to ‘anthracenylphenyl’}
Page 1010, P-68.3.1.3.3.2, example 2.
For 2-(2-phenyl-2-oxo-2λ5-diazenyl)naphthalen-1-yl preferred prefix)
read 2-(2-phenyl-2-oxo-2λ5-diazenyl)naphthalen-1-yl (preferred prefix)
Page 1011, P-68.3.1.3.4, second and third paragraph. [corrected 11 March 2020]
Move "The name 'carbazono' is not recommended." to the third paragraph
Page 1011, P-68.3.1.3.4, third paragraph, lines 1/2. [corrected 28 October 2016]
For ...The group H2N-NH-CO-N=NH– is named...
read ...The group H2N-NH-CO-N=N– is named...
Page 1011, P-68.3.1.3.4, last example. [corrected 28 October 2016]
For ethyl 3-(2-diazenecarbonylhydrazin-1-yl)propanoate (PIN)
read ethyl 3-[2-(diazenecarbonyl)hydrazin-1-yl]propanoate
add ethyl 3-(diazenecarbohydrazido)propanoate (PIN)
Page 1011, P-68.3.1.3.5, line 3. [corrected 20 March 2019]
For ...derivative of the parent hydride ‘diazene’; its...
read ...derivative of the parent hydride ‘hydrazine’; its...
Page 1011, P-68.3.1.3.5, structure 2. [modified 5 June 2019]
For(hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
replace structure with:
Page 1012, P-68.3.1.3.5.1, example 1. [corrected 20 March 2019]
For [hydrazinylidene(phenyl)methyl]phenyldiazene
read [phenyl(phenyldiazenyl)methylidene]hydrazine
Page 1012, P-68.3.1.3.5.1, example 2. [corrected 20 March 2019]
For [phenyl(phenylhydrazinylidene)methyl]diazene
read [diazenyl(phenyl)methylidene]phenylhydrazine
Page 1013, P-68.3.1.3.5.2, column 3 first name. [corrected 28 October 2016]
For hydrazinylidenemethyl)diazenyl
read (hydrazinylidenemethyl)diazenyl
Page 1014, P-68.3.1.3.5.2, example 1 on this page. [corrected 28 October 2016]
For 3-(2-{cyano-[(2-hydroxy-5-sulfophenyl)diazenyl]methylidene}hydrazinyl)-4-hydroxybenzene-1-sulfonic acid
read 3-(2-{cyano[(2-hydroxy-5-sulfophenyl)diazenyl]methylidene}hydrazinyl)-4-hydroxybenzene-1-sulfonic acid
for {not 3-(2-{cyano-[(2-hydroxy-5-sulfophenyl)hydrazinylidene]methyl}diazenyl)-4-hydroxybenzene-1-sulfonic acid
read {not 3-({cyano[(2-hydroxy-5-sulfophenyl)hydrazinylidene]methyl}diazenyl)-4-hydroxybenzene-1-sulfonic acid
Page 1014, P-68.3.1.3.5.2, example on this page. [corrected 5 July 2018]
Replace the structure with:
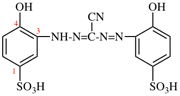
Page 1014, P-68.3.1.3.6, second paragraph, line 3. [corrected 28 October 2016]
For ...and telluro are used with the name carbadiazone in general nomenclature.
read ...and telluro are used with the name carbodiazone in general nomenclature.
Page 1014, P-68.3.1.3.6, box. [corrected 28 October 2016]
Delete the box.
Page 1015, P-68.3.1.4.1, box, line 1. [corrected 28 October 2016]
For ...systematic prefix names, i.e., triaazan-1-yl, etc.,
read ...systematic prefix names, i.e., triazan-1-yl, etc.,
Page 1015, P-68.3.1.4.1, box line 5
For ...names ‘epitrazano’, etc.
read ...names ‘epitriazano’, etc.
Page 1015, P-68.3.1.4.1, structure 3. [corrected 28 October 2016]
For triazan-1-yl- (preselected prefix)
read triazan-1-yl (preselected prefix)
Page 1015, P-68.3.1.4.1, structure 4. [corrected 28 October 2016]
For triaz-2-en-1-yl- (preselected prefix
read triaz-2-en-1-yl (preselected prefix)
for (not triaz[2]eno)
read (not triaz-2-eno)
Page 1015, P-68.3.1.4.1, last example on this page. [corrected 28 October 2016]
For (not 4-triazenobenzoic acid)
read [not 4-(triaz-2-eno)benzoic acid]
Page 1016, P-68.3.1.4.2, line 4. [corrected 28 October 2016]
For ... the group –N=NH-NH– is no longer recommended.
read ... the group –N=N-NH– is no longer recommended.
Page 1016, P-68.3.1.4.2, example 3. [corrected 28 October 2016]
Replace the structure with:
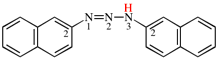
Page 1017, P-68.3.2.2, example 6. [corrected 1 July 2020]
For cyclotriphosphazene
read (not cyclotriphosphazene, which has only one double bond)
Page 1017, P-68.3.2.2, example 9. [modified 7 August 2019]
Add 3,5-dioxa-4-phospha-2-silaheptane
delete all of the Note:
replace the structure with:

Page 1018, P-68.3.2.3.1, example 6.
Delete [not (methylimino)phosphane] (repeat from example 5).
Page 1018, P-68.3.2.3.1, example 8. [corrected 5 June 2019]
For trimethyl-λ5-arsanimine (PIN)
read As,As,As-trimethyl-λ5-arsanimine (PIN)
Page 1018, P-68.3.2.3.1, last example.
For 2,4,6-triethoxy-1,3,5,2λ5,4λ5, 6λ5-triazatriphosphinane-2,4,6-trione (PIN)
read 2,4,6-triethoxy-1,3,5,2λ5,4λ5,6λ5-triazatriphosphinane-2,4,6-trione (PIN) [remove space in name]
Page 1019, P-68.3.2.3.2.1, example 3.
Delete (formerly pentamethyl holophosphate
Page 1019, P-68.3.2.3.2.1, example 5.
For naphthalen-2-ylarsane (PIN)
read (naphthalen-2-yl)arsane (PIN)
for naphthalen-2-ylarsine
read (naphthalen-2-yl)arsine
Page 1019, P-68.3.2.3.2.1, example 7.. [corrected 12 May 2021]
For ethyl(methyl)phenylphosphane (PIN)
read ethyl(methyl)(phenyl)phosphane (PIN)
for ethyl(methyl)phenylphosphine
read ethyl(methyl)(phenyl)phosphine
Page 1019, P-68.3.2.3.2.1, example 8.
For 1-benzofuran-2-ylphosphane (PIN)
read (1-benzofuran-2-yl)phosphane (PIN)
for 1-benzofuran-2-ylphosphine
read (1-benzofuran-2-yl)phosphine
Page 1019, P-68.3.2.3.2.1, example 9.
For thiophen-2-ylphosphane (PIN)
read (thiophen-2-yl)phosphane (PIN)
for thiophen-2-ylphosphine
read (thiophen-2-yl)phosphine
Page 1020, P-68.3.2.3.2.1, example 1 on this page.
For ethane-1,2-diylbis(dimethylphosphane) (PIN)
read (ethane-1,2-diyl)bis(dimethylphosphane) (PIN)
for ethane-1,2-diylbis(dimethylphosphine)
read (ethane-1,2-diyl)bis(dimethylphosphine)
Page 1020, P-68.3.2.3.2.1, example 2 on this page.
For butane-1,2,4-triyltris(phosphane) (PIN)
read (butane-1,2,4-triyl)tris(phosphane) (PIN)
for butane-1,2,4-triyltris(phosphine)
read (butane-1,2,4-triyl)tris(phosphine)
Page 1020, P-68.3.2.3.2.1, example 3 on this page.
For dibenzo[b,d]furan-3,7-diylbis(phosphane) (PIN)
read (dibenzo[b,d]furan-3,7-diyl)bis(phosphane) (PIN)
for dibenzo[b,d]furan-3,7-diylbis(phosphine)
read (dibenzo[b,d]furan-3,7-diyl)bis(phosphine)
Page 1020, P-68.3.2.3.2.1, example 4 on this page.
For 1,2-phenylenebis(arsane) (PIN)
read (1,2-phenylene)bis(arsane) (PIN)
for 1,2-phenylenebis(arsine)
read (1,2-phenylene)bis(arsine)
Page 1020, P-68.3.2.3.2.2, structure 3. [corrected 28 October 2016]
For –H2P-PH2– read –HP-PH–
Page 1020, P-68.3.2.3.2.2, example 2.. [modified 12 May 2021]
For (arsanylmethyl)diphenylphosphane (PIN)
read (arsanylmethyl)di(phenyl)phosphane (PIN)
for (arsinomethyl)diphenylphosphine
read (arsinomethyl)di(phenyl)phosphine
Page 1021, P-68.3.2.3.2.2, example 2 on this page.
For 4-[ethyl(methyl)phosphino]-1H-imidazole (PIN)
read 4-[ethyl(methyl)phosphino]-1H-imidazole
Page 1022, P-68.3.2.3.2.2, example 3 on this page. [modified 8 July 2017)
For methyl (dimethoxyphosphorimidoyl)acetate (PIN)
read methyl (P,P-dimethoxyphosphinimidoyl)acetate (PIN)
Page 1023, P-68.3.3, example 2 (structure 8 on this page)
For trimethylbusmuthine
read trimethylbismuthine
Page 1023, P-68.3.3, example 4. [corrected 5 June 2019]
For triphenyl-λ5-bismuthanimine (PIN)
read Bi,Bi,Bi-triphenyl-λ5-bismuthanimine (PIN)
Page 1023, P-68.3.3, last example on this page. [modified 20 March 2019]
For dichloro(triphenyl)-λ5-bismuthane (PIN)
read dichlorotri(phenyl)-λ5-bismuthane (PIN)
for dichloro(triphenyl)bismuthorane
read dichlorotri(phenyl)bismuthorane
for C6H5)3BiCl2
read (C6H5)3BiCl2
Page 1025, P-68.4.1.1, example 3 on this page.
For 1-methyl-3-phenyltriselane (PIN)
read methyl(phenyl)triselane (PIN)
Page 1025, P-68.4.1.1, example 4 on this page.
For 1,3-diphenyltriselane (PIN)
read diphenyltriselane (PIN)
Page 1025, P-68.4.1.2, example 1.
For methylenebis(trisulfane) (PIN)
read 1,1′-methylenebis(trisulfane) (PIN)
Page 1026, P-68.4.1.3, example 4. [corrected 27 March 2019]
Delete 1-(acetylpentathio)propan-1-one
Page 1026, P-68.4.2.1, example 4. [corrected 28 October 2016]
For 1-methyl-3-phenyldithioxane (PIN)
read methyl(phenyl)dithioxane (PIN)
Page 1027, P-68.4.2.1, example 2 on this page. [corrected 5 July 2018]
For (not oxydisulfanol)
read [not oxybis(sulfanol)]
Page 1028, P-68.4.2.4, example 2.
For [(methylperoxy)sulfanyl)]methane (PIN)
read [(methylperoxy)sulfanyl]methane (PIN)
Page 1028, P-68.4.2.4, example 4.
For [sulfanediylbis(oxy)]dibenzene
read 1,1′-[sulfanediylbis(oxy)]dibenzene
Page 1029, P-68.4.2.4, example 1 on this page.
For [(acetylsulfanyl)peroxy]propan-1-one (PIN)
read 1-[(acetylsulfanyl)peroxy]propan-1-one (PIN)
Page 1029, P-68.4.2.4, example 2 on this page. [corrected 20 March 2019]
For 1,1′-[disulfanediylbis(oxy)]dipropan-1-one (PIN)
read 1,1′-[disulfanediylbis(oxy)]di(propan-1-one) (PIN)
for 1,1′-[dithioperoxybis(oxy)]dipropan-1-one
read 1,1′-[dithioperoxybis(oxy)]di(propan-1-one)
Page 1030, P-68.4.3.1, example 2.
For (trimethoxy-λ4-sulfanyl)methane
read [(trimethoxy-λ4-sulfanyl)oxy]methane
delete (formerly tetramethyl orthosulfate; Rule D-6.92, ref. 1)
Page 1031, P-68.4.3.2, example 2 on this page. [corrected 20 March 2019]
For 1-[(ethaneseleninyl)selenonyl]ethane
read [(ethaneseleninyl)selenonyl]ethane
for 1-[(ethylseleninyl)selenonyl]ethane
read [(ethylseleninyl)selenonyl]ethane
Page 1031, P-68.4.3.3, example 2 . [corrected 20 March 2019]
For S,S-diphenyl-N-benzenesulfonylsulfimide
read S,S-diphenyl-N-(benzenesulfonyl)sulfimide
Page 1034, P-68.5.1, example 7.
For 1H-1λ3-bromirane (PIN)
read 1λ3-bromirane (PIN)
Page 1034, P-68.5.1, last example.
For 1λ3-benziodole (PIN)
read 1H-1λ3-benziodole (PIN)
Page 1035, P-68.5.3, example 1. [modified 21 April 2021]
For N-methylbromic amide(PIN)
read methylbromic amide (PIN) [add space before (PIN)]
Page 1035, P-68.5.3, example 2. [corrected 21 April 2021]
For N-ethylhypochlorous amide (PIN, see P-62.4)
read ethylhypochlorous amide (PIN, see P-62.4)
Page 1036, P-69.1, example 3. [corrected 5 June 2019]
For bromodiethenylstibane (PIN)
read bromodi(ethenyl)stibane (PIN)
Page 1038, P-69.2.3, example 1.
For (methyl)titanium
read trichlorido(methyl)titanium
Page 1038, P-69.2.3, example 2. [corrected 28 October 2016]
For (methyl)bis(triethylphosphane)platinum
read acetyl(methyl)bis(triethylphosphane)platinum
Page 1038, P-69.2.3, example 5. [corrected 9 April 2025]
For (4-carboxyphenyl)methylmercury
read (4-carboxyphenyl)(methyl)mercury
Page 1039, P-69.2.4, example 2. [corrected 21 August 2019]
For [(2,3,5,6-η4)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron
read [(2,3,5,6-η)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron
Page 1039, P-69.2.4, example 3.
For dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-ido](η5-cyclopenta-2,4-dien-1-ido)molybdenum
read dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopenta-2,4-dien-1-ido)molybdenum
for dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-yl](η5-cyclopenta-1,4-dien-1-yl)molybdenum
read dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-yl](η5-cyclopenta-2,4-dien-1-yl)molybdenum
for
dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-ido](η5-cyclopentadienido)molybdenum
read
dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopentadienido)molybdenum
Page 1040, P-69.2.5, example. [corrected 28 October 2016]
For {μ-[2(1-3,3a,8a-η):1(4–6-η)]azulene}-(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe)
read {μ-[2(1–3,3a,8a-η):1(4–6-η)]azulene}-(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe)
Page 1040, P-69.2.6, example 1.
For tricarbonyl{N,N-dimethyl-1-[2-(diphenylphosphanyl)-η6-phenyl]ethan-1-amine}chromium
read tricarbonyl{1-[2-(diphenylphosphanyl)-η6-phenyl]-N,N-dimethylethan-1-amine}chromium
Page 1041, P-69.2.6, example on this page. [modified 22 April 2020]
For [(1,2,5,6-η)-cycloocta-1,5-diene)](η6-phenyltriphenylboranuide)rhodium
read [(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)boranuido]rhodium
for [(1,2,5,6-η)-cycloocta-1,5-diene)](η6-phenyltriphenylborato)rhodium
read [(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)borato]rhodium
Page 1041, P-69.2.7, example 2. [modified 5 June 2019]
For 2-(osmocen-1-yl)ethanol (PIN)
read 2-osmocenylethan-1-ol (PIN)
for 1-(2-(hydroxyethyl)osmocene
read 1-(2-hydroxyethyl)osmocene
Page 1041, P-69.2.7, example 3. [modified 1 July 2020]
For N,N-dimethyl(vanadocen-1-yl)ethan-1-amine (PIN)
read N,N-dimethyl-1-vanadocenylethan-1-amine (PIN)
For 1-[1-(dimethylamino)ethyl]vanadocene
read [1-(dimethylamino)ethyl]vanadocene
Page 1042, P-69.2.7, example 2 on this page. [modified 20 March 2019]
For 1(1,1′),3(1,1′)-diferrocenacylotetraphane (PIN)
read 1,3(1,1′)-diferrocenacyclotetraphane (PIN)
Page 1042, P-69.2.7, example 3. [corrected 16 August 2016].
For 1,1′-(4-carboxybutane-1,3-diyl)ferrocene (PIN)
read 1,1′-(4-carboxybutane-1,3-diyl)ferrocene
for 3,5-(ferrocene-1,1′ -diyl)pentanoic acid
read 3,5-(ferrocene-1,1′-diyl)pentanoic acid (PIN) [space removed from name]
Page 1043, P-69.3, example 4. [corrected 28 October 2016]
For tetra-μ3-methanidotetralithium
read tetra-μ3-methanido-tetralithium
for tetra-μ3-methyltetralithium
read tetra-μ3-methyl-tetralithium
Page 1043, P-69.3, example 7. [corrected 28 October 2016]
For MgI(Me)]
read [MgI(Me)]
Page 1043, P-69.3, last example on this page. [corrected 28 October 2016]
For [(MgI(Me)]n
read [MgI(Me)]n
For poly[iodo(methyl)magnesium]
read poly[iodido(methyl)magnesium]
Page 1045, P-69.4, example 2 on this page. [modified 24.8.2017]
Delete 1-carbonyl-3,5-dimethyl-bis(triethylphosphane)iridine (Hantzsch-Widman type name)
for 1-carbonyl-3,5-dimethyl-bis(triethylphosphane)-1-iridabenzene
read 1-carbonyl-3,5-dimethyl-1,1-bis(triethylphosphane)-1-iridabenzene
Page 1045, P-69.4, example 3 on this page
For 9,9-[methylenebis(dimethylphosphane)-10H-9-platinaanthracene
read 9,9-[methylenebis(dimethylphosphane)]-10H-9-platinaanthracene
Page 1046, P-69.5.2, example. [corrected 28 October 2016]
For [(4-diphenylstibanyl)phenyl](phenyl)mercury
read [4-(diphenylstibanyl)phenyl](phenyl)mercury
replace the structure with:
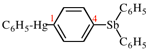
Page 1050, P-70.3.3.2.2, example [corrected 7 April 2021]
Delete this correction (leave as printed)
Page 1050, P-70.4, section (4) line 4.
For ...not ‘acetyl cation’. For CH3-C(O)-O+;...
read ...not ‘acetyl cation’, for CH3-C(O)+;...
Page 1050, P-70.4, section (4), lines 5-6. [modified 19 September 2018]
For ... and ‘methyliumyl’ not ‘methyl radical cation’, for CH4•–.
read ... and ‘methaniumyl’ not ‘methyl radical cation’, for CH4•+.
Page 1051, P-71.2.1.1, example 1.
Replace structure with:
•CH3
Page 1051, P-71.2.1.1, example 2.
Replace structure with:
•CH2-CH2-CH3
Page 1051, P-71.2.1.1, example 4.
Replace structure with:
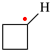
Page 1052, P-71.2.1.2, example 5. [modified 12 June 2019]
For tert-butoxy(triphenyl)-λ5-phosphanyl (PIN)
read tert-butoxytri(phenyl)-λ5-phosphanyl (PIN)
for [(2-methylpropan-2-yl)oxy]triphenyl-λ5-phosphanyl
read [(2-methylpropan-2-yl)oxy]tri(phenyl)-λ5-phosphanyl
for (1,1-dimethylethoxy)triphenyl-λ5-phosphanyl
read (1,1-dimethylethoxy)tri(phenyl)-λ5-phosphanyl
Page 1053, P-71.2.1.3, example. [corrected 19 September 2018]
For anthracene-9-eliumuide
read anthracen-9-eliumuide
Page 1053, P-71.2.2.1, line 10.
For ... aminylene, silylene, and carbyne, ...
read ... aminylene, and carbyne, ...
Page 1054, P-71.2.2.1, example 4.
For diphenylnethanediyl
read diphenylmethanediyl
Page 1057, P-71.2.6, example 3.
For naphthalen-4a(8aH)-yl
read naphthalen-4a(8aH)-yl
Page 1057, P-71.2.6, example 4. [corrected 3 October 2018]
For (C60-Ih)[5,6]fulleren-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-yl (PIN)
for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-yl
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-yl
Page 1058, P-71.3.1, example 2. [corrected 12 June 2019]
For dimethyloxo-λ5-phosphanyl
read dimethyl(oxo)-λ5-phosphanyl
Page 1058, P-71.3.1, example 6. [modified 7 August 2019]
For 1,4-phenylenebis(oxo-λ4-sulfanyl)
read (1,4-phenylene)bis(oxo-λ4-sulfanyl)
replace the structure with:

Page 1058, P-71.3.1, example 8. [corrected 7 August 2019]
For 1,4-phenylenebis(oxomethyl)
read (1,4-phenylene)bis(oxomethyl)
Page 1060, P-71.3.3, example 1. [modified 7 August 2019]
For (1) ethane-1,2-bis(aminyl) (PIN)
read (1) (ethane-1,2-diyl)bis(aminyl) (PIN)
Page 1060, P-71.3.3, example 3. [corrected 7 August 2019]
For (2) benzene-1,2-dicarbonylbis(azanyl)
read (2) (benzene-1,2-dicarbonyl)bis(azanyl)
Page 1061, P-71.3.4, line 9.
For ...phenoxy, and aminooxyl, which...
read ...phenoxy, and aminoxyl, which...
Page 1061, P-71.3.4, example 2.
For (1) chloroacetoxyl (PIN)
read chloroacetoxyl
add (1) (chloroacetyl)oxyl (PIN)
Page 1062, P-71.3.4, example 2 on this page.
For butanoyloxyl
read butanoyloxyl (PIN)
for butanoyloxidanyl (PIN)
read butanoyloxidanyl
Page 1062, P-71.3.4, example 4 on this page.
For acetylselanyl (PIN)
read methylselanyl (PIN)
Page 1062, P-71.3.4, last example.
For [chloro(ethanethioyl)]sulfanyl (PIN)
read (chloroethanethioyl)sulfanyl (PIN)
Page 1062, P-71.4, example 1.
For cyclopropane-1,2-diyldimethyl (PIN)
read (cyclopropane-1,2-diyl)dimethyl (PIN)
Page 1062, P-71.4, example 2.
For naphthalene-2,6-diylbis(disulfanyl) (PIN)
read (naphthalene-2,6-diyl)bis(disulfanyl) (PIN)
Page 1062, P-71.4, example 3.
Replace structure with:
•O-C(CH3)2-CH2-C(CH3)2-O•
Page 1062, P-71.4, example 4.
For (1) cyclobutane-1,3-diylbis(peroxyl) (PIN)
read (1) (cyclobutane-1,3-diyl)bis(peroxyl) (PIN)
for (2) cyclobutane-1,3-diylbis(dioxidanyl)
read (2) (cyclobutane-1,3-diyl)bis(dioxidanyl)
Page 1063, P-71.5, example 4. [corrected 19 September 2018]
For oxylcarboxyl (preferred prefix)
read oxylcarbonyl (preferred prefix)
Replace the structure with:
—CO-O•
Page 1063, P-71.5, line before examples. [corrected 21 April 2021]
Delete this line
Page 1063, P-71.5, example 2. [corrected 21 April 2021]
For oxyl (preselected name)
read ylooxidanyl (preselected name)
for ylooxidanyl
read ylooxy
Page 1064, P-71.7 (a) example.
Add [not 4-(1,2-diyloethyl)cyclohexyl; ethane has two radical centers, cyclohexane only has one]
Page 1064, P-71.7 (b), example. [corrected 10 October 2018]
Replace the structure with:
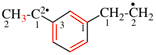
Page 1064, P-71.7 (c), example.
Replace the structure with:
•CH2-C(CH3)2-O•
Page 1065, P-71.7 (d) (1) example. [modified 21 April 2021]
For (3-oxylbenzoyl)oxyl (PIN)
read [3-(ylooxidanyl)benzoyl]oxyl (PIN)
add {not 3-[(ylooxidanyl)carbonyl]phenoxyl; ylooxidanyl is senior to phenoxyl}
replace the structure with:
Page 1065, P-71.7 (d), example 2 on this page. [corrected 26 September 2018]
Replace the structure with:
Page 1066, P-72.2.1, example 3.
For oxophenyl-λ4-sulfanide (PIN)
read oxo(phenyl)-λ4-sulfanide (PIN)
Page 1067, P-72.2.2.1, example 1.
Replace structure with:
(NC)3C –
Page 1068, P-72.2.2.1.1, example 1. [corrected 19 September 2018]
For 1,2-dihydropyridine-1-ide
read 1,2-dihydropyridin-1-ide
Page 1068, P-72.2.2.1.1, example 2. [corrected 19 September 2018]
For 1-methyl-1,2-dihydro-1-benzoazocine-2,2-diide
read 1-methyl-1,2-dihydro-1-benzazocine-2,2-diide
Page 1068, P-72.2.2.1.1, example 3.
Delete X = Y = –
delete naphthalene-1,4-diide (PIN)’
for 1,4-dihydronaphthalene-1,4-diide
read 1,4-dihydronaphthalene-1,4-diide (PIN)
Replace structure with:
Page 1068, P-72.2.2.1.1, example 4. [corrected 3 October 2018]
For (C60-Ih)[5,6]fulleren-1(9H)-ide (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ide (PIN)
for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ide
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ide
Page 1070, P-72.2.2.2.1.1, example 2 on this page. [corrected 28 October 2016]
For ethane-OS-(thioperoxoate) (PIN)
read ethane(OS-thioperoxoate) (PIN)
Page 1071, P-72.2.2.2.1.2, example.
For phenyl hydrogen phosphonate (PIN)
read phenyl hydrogen phosphate (PIN)
Page 1071, P-72.2.2.2.2, third paragraph. [corrected 28 October 2016]
For The traditional names methoxide, ethoxide, propoxide (but not isopropoxide), butoxide, tert-butoxide, phenoxide and aminoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, (CH3)3C-O–, C6H5-O– and H2N-O–, are retained as preferred IUPAC names.
read The traditional names methoxide, ethoxide, propoxide, butoxide, tert-butoxide, phenoxide and aminoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, (CH3)3C-O–, C6H5-O– and H2N-O– are retained as preferred IUPAC names. tert-Butoxide cannot be substituted. Isopropoxide, (CH3)2CH-O–, is retained for general nomenclature but cannot be substituted.
Page 1072, P-72.2.2.2.2, example 5 on this page. [corrected 27 March 2019]
For ethylsulfanyl)oxidanide
read (ethylsulfanyl)oxidanide
for (not benzenesulfenate; sulfenic acids ...
read (not ethanesulfenate; sulfenic acids ...
Page 1072, P-72.2.2.2.2, example 6. [corrected 7 August 2019]
For ethane-1,2-diylbis(dioxidanide)
read (ethane-1,2-diyl)bis(dioxidanide)
Page 1072, P-72.2.2.2.2, example 7. [corrected 7 August 2019]
For 1,4-phenylenebis(disulfanide)
read (1,4-phenylene)bis(disulfanide)
Page 1072, P-72.2.2.2.2, example 8. [corrected 7 August 2019]
For 1,4-phenylenebis(oxy)bis(sulfanide)
read (1,4-phenylene)bis(oxy)bis(sulfanide)
Page 1073, P-72.2.2.2.3, example 3. [corrected 7 August 2019]
For ethane-1,2-diylbis(amide)
read (ethane-1,2-diyl)bis(amide)
Page 1073, P-72.2.2.2.4, example 1. [corrected 19 September 2018]
For (2-methylpropan-2-y)trioxidanide
read (2-methylpropan-2-yl)trioxidanide
Page 1073, P-72.2.2.2.4, example 2.
For 1-oxoethanide
read 1-oxoethan-1-ide
Page 1073, P-72.2.2.2.4, example 4. [corrected 26 September 2018]
Replace the structure with:
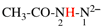
Page 1077, P-72.4, example 1 on this page.
For 6λ5-phosphanuidaspiro[5.5]nonan
read 6λ5-phosphanuidaspiro[5.5]undecane
for
(not 6λ5-phosphataspiro[5.5]nonan)
read (not 6λ5-phosphataspiro[5.5]undecane)
Page 1077, P-72.5.1.1, example 1. [modified 7 August 2019]
For 1,4-phenylenebis(phosphanide) (PIN)
read (1,4-phenylene)bis(phosphanide) (PIN)
Delete second 1,4-phenylenebis(phosphanide) (PIN)
Page 1078, P-72.5.3, example 1. [corrected 10 October 2018]
Replace the structure with:
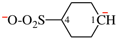
Page 1080, P-72.7 (b), example.
Page 1080, P-72.7 (b) example. [modified 21 April 2021]
Replace example with
2-(2-carboxylatoethyl)-1,1,5,5-tetracyanopentane-1,5-diide (PIN)
[not 1,1-dicyano-4-(dicyanomethanidyl)heptan-1-id-7-oate; diide is senior to idoate]
replace structure with:
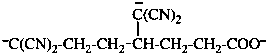
Page 1080, P-72.7 (c), example. [corrected 7 November 2018]
For (2-phosphanidylethyl))arsanuide
read (2-phosphanidylethyl)arsanuide
Page 1081, P-72.7 (e), example 2.
For oxidonaphthalene-2-carboxylate (PIN)
read 3-oxidonaphthalene-2-carboxylate (PIN)
Page 1082, P-72.8.2, example 2. [corrected 17 June 2020]
For 1λ6, 3λ6-dithiocane-1,3-diide (PIN)
read 1λ6,3λ6-dithiocane-1,3-diide (PIN)
for 1λ4, 3λ4-dithiocane-1,3-diuide
read1λ4,3λ4-dithiocane-1,3-diuide
for 1λ4, 3λ6-dithiocan-3-id-1-uide
read (not 1λ4,3λ6-dithiocan-3-id-1-uide; identical groups must not be named differently within a name, if possible)
[remove space in names]
Page 1083, P-73.1.1.1, example 2. [corrected 27 March 2019]
For chloro(trimethyl)phosphonium
read chlorotri(methyl)phosphonium (PIN)
for chloro(trimethyl)phosphanium (PIN)
read chlorotri(methyl)phosphanium (PIN)
Page 1084, P-73.1.1.2, line 1 on this page.
For ...multiplying locants ‘di’,...
read ...multiplying prefixes ‘di’,...
Page 1085, P-73.1.1.2, example 1 on this page.
For 1,1,3,3-tetraphenyl-4,5-dihydro-3H-1λ5,2,3-triphosphol-3-ium (PIN)
read 1,1,3,3-tetraphenyl-4,5-dihydro-1H-1,2,3λ5-triphosphol-1-ium (PIN)
Change structure to:
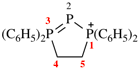
Page 1085, P-73.1.1.2, example 4 on this page, move to P-73.1.2.1. [modified 30.6.2017)
For 1,1,1,5,5,5-hexamethyltrisilazane-2,4-diium (PIN)
read N,N′-bis(trimethylsilyl)silanebis(aminium) (PIN)
Page 1086, P-73.1.2.1, example 2. [corrected 12 June 2019]
For benzoyltrimethylammonium
read benzoyltri(methyl)ammonium
Page 1086, P-73.1.2.1, example 5. [corrected 12 June 2019]
For (1,4-dithian-2-yl)trimethylammonium
read (1,4-dithian-2-yl)tri(methyl)ammonium
Page 1086, P-73.1.2.1, example 7.
For N,N,N-trimethylbenzenaminium (PIN)
read N,N,N-trimethylbenzenaminium
add N,N,N-trimethylanilinium (PIN)
Page 1087, P-73.1.2.1, example 5 on this page.
For [1-(oxidaniumyl)ethylidene]oxidanium (PIN)
read (1-oxidaniumylethylidene)oxidanium (PIN)
Page 1087, P-73.1.2.1, example 6 on this page. [corrected 12 June 2019]
For (cyclohexanecarbonyl)dimethyloxidanium (PIN)
read (cyclohexanecarbonyl)di(methyl)oxidanium (PIN)
for (cyclohexanecarbonyl)dimethyloxonium
read (cyclohexanecarbonyl)di(methyl)oxonium
Page 1087, P-73.1.2.1, example 7 on this page. [corrected 27 March 2019]
For (1-hydroxyethylidene)(dimethyl)azanium (PIN)
read (1-hydroxyethylidene)di(methyl)azanium (PIN)
for (1-hydroxyethylidene)(dimethyl)ammonium
read (1-hydroxyethylidene)di(methyl)ammonium
Page 1088, P-73.1.2.1, example 1 on this page. [corrected 12 June 2019]
For benzoyldimethylsulfanium
read benzoyldi(methyl)sulfanium
for benzoyldimethylsulfonium
read benzoyldi(methyl)sulfonium
Page 1088, P-73.1.2.1, example 2 on this page.
For benzoyldioxidanium (PIN)
read 2-benzoyldioxidan-1-ium (PIN)
Page 1088, P-73.1.2.2, right structural formula. [corrected 12 June 2019]
Replace the structure with:
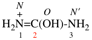
Page 1088, P-73.1.2.2, example 1. [modified 11 March 2020]
For N-(methylamino)phenoxymethylidene]methanaminium
read N-[(methylamino)phenoxymethylidene]methanaminium
Replace the structures with:
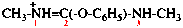
Page 1089, P-73.2.1, example 2.
Add phenylium
Page 1089, P-73.2.1, example 3.
For 1-oxoethylium (PIN)
read acetylium (PIN)
Page 1089, P-74.2.2, line 4.
For P-73.2.2.2 Cationic centres on characteristic groups
read P-73.2.2.2 ‘Added indicated hydrogen’
P-73.2.2.3 Diazonium ions
Page 1091, P-73.2.2.2, line 4.
For ... Such a radical can also...
read ... Such a cation can also...
Page 1091, P-73.2.2.2, line 10.
For ...for the radical center...
read ...for the cationic center...
Page 1092, P-73.2.2.2, example 1 on this page.
For X = + naphthalen-4a(8aH)-ylium (PIN)
read naphthalen-4a(8aH)-ylium (PIN)
for Y = H 4a,8a-dihydronaphthalen-4a-ylium
read 4a,8a-dihydronaphthalen-4a-ylium
Replace structure with:
Page 1092, P-73.2.2.2, example 2 on this page. [corrected 3 October 2018, modified 10 October 2018]
For (C60-Ih)[5,6]fulleren-1(9H)-ylium (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ylium (PIN)
for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ylium
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ylium
replace the structure with:
Page 1092, P-73.2.2.3, example 3. [corrected 7 August 2019]
For 1,4-phenylenebis(diazenylium) (PIN)
read (1,4-phenylene)bis(diazenylium) (PIN)
Page 1093, P-73.2.3.1, example 6. [corrected 17 June 2020]
For methylphosphonoylium (PIN)
read methylphosphonobis(ylium) (PIN)
Page 1093, P-73.2.3.2, example 3.
Replace structure with:
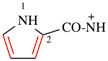
Page 1094, P-73.2.3.3, paragraph above examples, lines 1/2. [corrected 1 November 2023]
For The names methoxylium, ethoxylium, propoxylium, butoxylium, phenoxylium, and aminoxylium are retained as preferred IUPAC names....
read The names methoxylium, ethoxylium, propoxylium, butoxylium, and phenoxylium are retained as preferred IUPAC names, and aminoxylium as a preselected name....
Page 1094, P-73.2.3.3, example 3.
For chloracetoxylium (PIN)
read chloroacetoxylium
add (chloroacetyl)oxylium (PIN)
Page 1094, P-73.2.3.3, example 5. [corrected 12 June 2019]
For dimethylaminoxylium (PIN)
read N-methylmethanaminoxylium (PIN)
Page 1094, P-73.2.3.3, last example.
Replace the structure with:
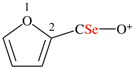
Page 1095, P-73.2.3.4, example 2 on page. Replace the structure with:
CH3–CH2–S–O+
Page 1095, P-73.2.3.4, example 5 on this page. [corrected 19 September 2018]
For 2-benzoyldiazane-1-ylium
read 2-benzoyldiazan-1-ylium
Page 1095, P-73.2.3.4, example 6.
For (not ethyltelluorperoxylium)
read (not ethyltelluroperoxylium)
Page 1095, P-73.3.1, example 1.
Add (not 3H-thienylium)
Page 1096, P-73.3.1, example 2 on this page. [corrected 12 June 2019]
For 1λ3-benzoiodol-1-ylium
read 1λ3-benziodol-1-ylium
Page 1096, P-73.3.1, last example.
Add (not 3H-thienylium)
Page 1097, P-73.3.2, Table 7.5, structure 3. [corrected 27 March 2019]
For E = S 2λ4-benzothiopyran-1-ylium (PIN)
read E = S 2λ4-benzothiopyran-2-ylium (PIN)
for E = Se 2λ4-benzoselenopyran-1-ylium (PIN)
read E = Se 2λ4-benzoselenopyran-2-ylium (PIN)
for E = Te 2λ4-benzotelluropyran-1-ylium (PIN)
read E = Te 2λ4-benzotelluropyran-2-ylium (PIN)
Page 1097, P-73.3.2, Table 7.5, structure 4. [modified 30.6.2017)
For 2H-furylium (PIN)
read 2H-1λ4-furan-1-ylium (PIN)
for 2H-thienylium (PIN)
read 2H-1λ4-thiophen-1-ylium (PIN)
for 2H-selenofuranylium (PIN)
read 2H-1λ4-selenophen-1-ylium (PIN)
for 2H-tellurofuranylium (PIN)
read 2H-1λ4-tellurophen-1-ylium (PIN)
Page 1097, P-73.3.2, Table 7.5, structure 5.
For E = O flavylium (PIN)
read E = O flavylium
for E = S thioflavylium (PIN)
read E = S thioflavylium
for E = Se selenoflavylium (PIN)
read E = Se selenoflavylium
for E = Te telluroflavylium (PIN)
read E = Te telluroflavylium
Page 1099, P-73.4, example 1 on this page. [modified 30 March 2022]
For 2-ethoxy-N-{2-[(2-ethoxyethyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
read 2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
for 2-ethoxy-N-{2-[(2-ethoxyethyl)sulfanyl]ethyl}-N-methylethanamine
read 2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfanyl]ethyl}-N-methylethanamine
for N-(2-ethoxyethyl)-2-[(2-ethoxyethyl)sulfanyl]-N-methylethanamine
read N-(2-ethoxyethyl)-2-[(2-ethoxyethyl)(methyl)sulfanyl]-N-methylethanamine
Page 1099, P-73.4, example 2 on this page.
Replace the structure with:
Page 1099, P-73.4, last example on this page.
For 5H-5λ5-arsaspiro[4.4]nonan-5-ylium (PIN)
read 5λ5-arsaspiro[4.4]nonan-5-ylium (PIN)
Page 1100, P-73.4, example 3 on this page. [corrected 10 October 2018]
Replace the structure with:
Page 1101, P-73.5.1.1, example 1. [corrected 7 August 2019]
For 1,4-phenylenebis(phosphanium) (PIN)
read (1,4-phenylene)bis(phosphanium) (PIN)
for 1,4-phenylenebis(phosphonium)
read (1,4-phenylene)bis(phosphonium)
Page 1101, P-73.5.1.1, example 3. [corrected 12 June 2019]
Delete 2,2′-benzene-1,3-diyldi(propan-2-ylium)
Page 1101, P-73.5.1.2, example 1. [corrected 7 August 2019]
For ethane-1,2-diylbis(oxylium) (PIN)
read (ethane-1,2-diyl)bis(oxylium) (PIN)
for ethane-1,2-diylbis(oxidanylium)
read (ethane-1,2-diyl)bis(oxidanylium)
Page 1101, P-73.5.1.2, example 3. [corrected 7 August 2019]
For pentane-2,4-diylidenebis(oxidanium) (PIN)
read (pentane-2,4-diylidene)bis(oxidanium) (PIN)
for pentane-2,4-diylidenebis(oxonium)
read (pentane-2,4-diylidene)bis(oxonium)
Page 1102, P-73.5.1.2, example 4 on this page. [corrected 7 August 2019]
For pyridine-2,6-diylbis(sulfanylium) (PIN)
read (pyridine-2,6-diyl)bis(sulfanylium) (PIN)
for pyridine-2,6-diylbis(thioxylium)
read (pyridine-2,6-diyl)bis(thioxylium)
Page 1102, P-73.5.1.2, example 5 on this page. [corrected 7 August 2019]
For 1,4-phenylenebis(dioxo-λ6-sulfanylium) (PIN)
read (1,4-phenylene)bis(dioxo-λ6-sulfanylium) (PIN)
Page 1105, P-73.6, example 5.
For sulfoniumyl
read selenoniumyl
Page 1105, P-73.6, example 8. [corrected 17 June 2020]
For dimethylazaniumylidene (preferred prefix)
read N-methylmethanaminiumylidene (preferred prefix)
Page 1106, P-73.7.1 (c), example 1. [modified 12 June 2019]
For trimethyl[6-(dimethylsulfaniumyl)hexyl]phosphanium (PIN)
read [6-(dimethylsulfaniumyl)hexyl]tri(methyl)phosphanium (PIN)
Page 1106, P-73.7.1 (c), example 2. [modified 11 November 2020]
For trimethyl[(6-dimethylsulfaniumyl)hexyl]ammonium (PIN)
read 6-(dimethylsulfaniumyl)-N,N,N-trimethylhexan-1-aminium (PIN)
Page 1107, P-73.8.2, example 2.
For λ6,3λ6-dithiocane-1,3-bis(ylium)
read 1λ6,3λ6-dithiocane-1,3-bis(ylium)
for 1λ6,3λ4-dithiocan-3-ium-1-ylium
read (not 1λ6,3λ4-dithiocan-3-ium-1-ylium; identical groups, if possible, must not be named differently within a name)
Page 1108, P-74.1.1, example 1.
For 1,2,2-trimethylhydrazin-2-ium-1-ide (PIN)
read 1,2,2,2-tetramethylhydrazin-2-ium-1-ide (PIN)
replace structure with:
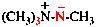
Page 1109, P-74.1.1, example 1 on this page. [corrected 9 January 2019]
For 5H-11λ5-5H-indolo[2,3-b]quinolizin-11-ylium-5-ide (PIN)
read 5H-11λ5-indolo[2,3-b]quinolizin-11-ylium-5-ide (PIN)
Page 1109, P-74.1.1, example 3 on this page.. [modified 12 May 2021]
For 6,6-dihydroxy-6,11-dihydro-5λ5-benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide (PIN)
read 6,6-dihydroxy-6,11-dihydro-5λ5-benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide
add 6,6-dihydroxy-6,11-dihydro-5λ5-[1,3]benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide (PIN)
Page 1109, P-74.1.1, example 4 on this page. [modified 21 November 2018]
For 2-methyl-4-oxo-3,4-dihydro-1H-2-selenobenzopyran -2-ium-3-ide (PIN)
read 2-methyl-4-oxo-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-ide (PIN)
for not 2-methyl-3,4-dihydro-1H-2-selenobenzopyran-2-ium-3-id-4-one
read not 2-methyl-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-id-4-one
for 2-methyl-4-oxo-3,4-dihydro-1H-isoselenochromen-2-ium-3-ide (PIN)
read 2-methyl-4-oxo-3,4-dihydro-1H-isoselenochromen-2-ium-3-ide
Page 1110, P-74.1.1, example 1 on this page. [corrected 14 November 2018]
For 5λ5,7λ5-spiro[1,3,2-diazaborolo[3,4-a:5,1-a′]dipyridine-6,10′-phenoxaborinine]-5,7-bis(ylium)-6-uide
read 5λ5,7λ5-spiro[[1,3,2]diazaborolo[3,4-a:5,1-a′]dipyridine-6,10′-phenoxaborinine]-5,7-bis(ylium)-6-uide
Page 1110, P-74.1.2, example 1. [modified 11 November 2020]
Replace the structure with:
Page 1111, P-74.1.3, example 1. [modified 27 March 2019]
For trimethyl[2-(methyldiphenylphosphaniumyl)ethenyl]boranuide (PIN)
read trimethyl{2-[methyldi(phenyl)phosphaniumyl]ethen-1-yl}boranuide (PIN)
for trimethyl[2-(methyldiphenylphosphoniumyl)ethenyl]boranuide
read trimethyl{2-[methyldi(phenyl)phosphoniumyl]ethen-1-yl}boranuide
Page 1111, P-74.1.3, example 2. [corrected 17 June 2020]
For (trimethylazaniumyl)acetate (PIN)
read (N,N-dimethylmethanaminiumyl)acetate (PIN)
Page 1111, P-74.1.3, example 3. [modified 17 June 2020]
For N-methyl-N′-(methylboranuidyl)boranuidediylbis(aminium) (PIN)
read methyl{[(methanaminiumyl)boranuidyl]azaniumyl}boranuide (PIN)
for [not 1-methyl-3-(methylazaniumyl)diborazan-2-ium-1,3-diide;
read [not 1-methyl-3-(methanaminiumyl)diborazan-2-ium-1,3-diide;
Replace the structure with:
Page 1112, P-74.2.1.1.1, example. [corrected 17 June 2020]
For 2-(trimethylazaniumyl)propan-2-ide (PIN)
read 2-(N,N-dimethylmethanaminiumyl)propan-2-ide (PIN)
Page 1112, P-74.2.1.1.2, example. [modified 12 June 2019]
For trimethyl(isopropylidene)phosphorane
read isopropylidenetri(methyl)phosphorane
Page 1113, P-74.2.1.2, example.
For N,N-dimethylmethanamine oxide (PIN)
read N,N-dimethylmethanamine N-oxide (PIN)
for trimethyl)amine oxide
read (trimethyl)amine oxide
Page 1114, P-74.2.1.5, example.
Replace structure with:
Page 1116, P-74.2.2.1.2, structures.
For R-N––+(R)=CR2 ↔
R-N=N+(R)-C–R2
read R-N––N+(R)=CR2 ↔ R-N=N+(R)-C–R2
Page 1116, P-74.2.2.1.3, example. [corrected 17 June 2020]
For 2-[methyl(propan-2-ylidene)azaniumyl]propan-2-ide (PIN)
read 2-(N-methylpropan-2-iminiumyl)propan-2-ide (PIN)
Page 1116, P-74.2.2.1.4, example.
For diphenyldiazeniumyl)oxidanide
read (diphenyldiazeniumyl)oxidanide
Page 1117, P-74.2.2.1.6, structures.
For
R2C––O+=O ↔ R2C––O-O+
read R2C––O+=O ↔ R2C=O+–O–
Page 1117, P-74.2.2.1.8, example 2. [modified 27 March 2019]
For 1λ4-thiophen-1-one (PIN)
read 1H-1λ4-thiophen-1-one (PIN)
for 1-oxo-1λ4-thiophene
read 1-oxo-1H-1λ4-thiophene
Page 1118, P-74.2.2.1.8, example on this page. [modified 27 March 2019]
For 1λ4,2-thiazin-1-one (PIN)
read 1H-1λ4,2-thiazol-1-one (PIN)
for 1,2-thiazine 1-oxide
read 1,2-thiazole 1-oxide
for 1,2-thiazine S-oxide
read 1,2-thiazole S-oxide
Page 1118, P-74.2.2.1.9 example.
For N-methylpropan-2-imine oxide
read N-methylpropan-2-imine N-oxide
Page 1118, P-74.2.2.2, structures.
For X≡N+–Z– ↔ –X=N+=Z ↔ –X=N-Z+ ↔ X-N=Z,
read X≡N+–Z– ↔ X–=N+=Z ↔ X–=N–Z+,
for where X = C or O; and Z = C, N or O
read where X = C, N or O; and Z = C, N or O
Page 1119, P-74.2.2.2.1.3, heading.
For P-74.2.2.2 Nitrile ylides.
read P-74.2.2.2.1.3 Nitrile ylides.
Page 1119, P-74.2.2.2.1.2, example 1.
Add (ethylidyneazaniumyl)oxidanide
Page 1119, P-74.2.2.2.1.2, example 2.
For (ethylidyneazaniumyl)oxidanide
read (ethylidyneazaniumyl)sulfanide
add acetonitrile sulfide (PIN)
Page 1119, P-74.2.2.2.1.3, example. [corrected 17 June 2020]
For 2-(ethylidyneazaniumyl)propan-2-ide (PIN)
read 2-(acetonitriliumyl)propan-2-ide (PIN)
Page 1119, P-74.2.2.2.2, example. [modified 3.7.2017)
For phenyltriazadien-2-ium
read 3-phenyltriazadien-2-ium-1-ide
Page 1120, P-74.2.2.3, structures.
For X–C=Z ↔ +X=C–Z–
read
X2•–C=Z ↔ X+=C–Z–
Page 1121, P-74.2.2.3.4. line 1. [corrected 26 September 2018]
For RR′=CR′′-C2•-R′′′
read RR′C=CR′′-C2•-R′′′
Page 1121, P-74.2.3, example 1. [modified 27 March 2019]
For 2-{4-[oxido(diphenyl)phosphaniumyl]phenyl}propane-1,3-diyl (PIN)
read 2-{4-[oxidodi(phenyl)phosphaniumyl]phenyl}propane-1,3-diyl (PIN)
for 2-{4-[oxido(diphenyl)phosphoniumyl]phenyl}propane-1,3-diyl
read 2-{4-[oxidodi(phenyl)phosphoniumyl]phenyl}propane-1,3-diyl
replace the structure with:
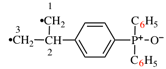
Page 1121, P-74.2.3, example 2. [corrected 26 September 2018]
Replace the structure with:
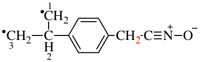
Page 1123, P-75.2.1, example 5. [corrected 26 September 2018]
Replace the structure with:
Page 1124, P-75.2.3, example 1. [modified 11 November 2020]
For (triethylazaniumyl)boranuidyl (PIN)
read (N,N-diethylethanaminiumyl)boranuidyl (PIN)
Page 1124, P-75.2.3, example 2. [modified 19.7.2017)
Replace structure with:
Page 1125, P-75.2.4, example 1 on this page. [corrected 10 October 2018]
Replace the structure with:
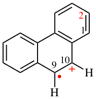
Page 1125, P-75.2.4, example 2 on page.
For 1-ethyl-2-oxopyridin-1-ium-1(2H)-yl (P
read 1-ethyl-2-oxopyridin-1-ium-1(2H)-yl (PIN)
Page 1125, P-75.2.4, example 3 (X = • ; Y = +) on this page. [corrected 3 October 2018]
For (C60-Ih)[5,6]fulleren-9-ylium-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-9-ylium-1(9H)-yl (PIN)
for 1,9-dihydro(C60-Ih)[5,6]fulleren-1,9-diyl
read 1,9-dihydro(C60-Ih)[5,6]fulleren-9-ylium-1-yl
Page 1125, P-75.2.4, example 3 (X = • ; Y = –) on this page. [corrected 3 October 2018]
For (C60-Ih)[5,6]fulleren-9-id-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-9-id-1(9H)-yl (PIN)
for 1,9-dihydro(C60-Ih)[5,6]fulleren-9-id-1-yl
read 1,9-dihydro(C60-Ih)[5,6]fulleren-9-id-1-yl
Page 1125, P-75.3.1 example 2. [corrected 26 September 2018]
Replace the structure with:
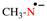
Page 1125, P-75.2.4, example 3 on this page. [corrected 10 October 2018]
Replace the structure with:
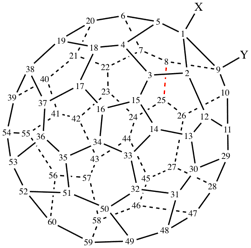
Page 1126, P-75.3.2, example 4.
For 2-(benzenesulfonyl)diazane-1-id-1-yl
read 2-(benzenesulfonyl)diazan-1-id-1-yl
Page 1126, P-75.3.2, example 3.
Replace structure with:
CH3-CO-N•–
Page 1126, P-75.3.2, example 4.
Replace structure with:
Page 1126, P-75.4, example 1.
For 2-oxidaniumylidyneethyl(PIN)
read (oxidaniumylidyne)ethyl (PIN)
Page 1126, P-75.4, example 2.
For ethoxy(oxido)benzyl
read α-ethoxy-α-oxidobenzyl
Page 1127, P-77.1.1, example 1.
For benzenaminium chloride (PIN)
read benzenaminium chloride
add anilinium chloride (PIN)
Page 1127, P-77.1.1, example 1,
Replace structure with:
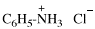
Page 1127, P-77.1.1, example 2,
Replace structure with:

Page 1127, P-77.1.1, example 3,
Replace structure with:

Page 1127, P-77.1.2, line 2.
For ... Adduct names (see P-77.1.2) may be...
read ... Adduct names (see P-77.1.3) may be...
Page 1128, P-77.1.3 (1), line 1. (corrected 4 December 2019)
For as adducts, leading to preferred IUPAC names;
read as adducts. The names of these adducts are preferred IUPAC names only when acid components of the adducts are organic compounds.
Page 1128, P-77.1.3, example. [modified 21 November 2018]
For (a) N,N-dimethyl-1,3-thiazolidin-2-amine—sulfuric acid (2/1) (PIN)
read (1) N,N-dimethyl-1,3-thiazolidin-2-amine—sulfuric acid (2/1) (PIN)
for (b) bis(N,N-dimethyl-1,3-thiazolidin-2-amine) sulfate
read (2) bis(N,N-dimethyl-1,3-thiazolidin-2-amine) sulfate
Page 1128, P-77.2.1, example 1. [corrected 21 November 2018]
For sodium methanolate (PIN)
read sodium methanolate
for sodium methoxide
read sodium methoxide (PIN)
delete a retained name) and methanolate.
Page 1128, P-77.2.1, example 2.
For sodium 4-methylbenzenethiolate (PIN)
read sodium 4-methylbenzene-1-thiolate (PIN)
Page 1129, P-77.3.1, example. [corrected 10 October 2018]
For H3-CO-O–
read CH3-CO-O–
Page 1130, P-80, lines 3/4. [corrected 7 August 2019]
For ...see ref. 29). Comparative examples of the application of these rules are given in Table 8.1, below). This...
read ...see ref. 29). Examples of isotopic nuclides for hydrogen are given in Table 8.1, below. This...
Page 1132, P-82.2.1, example 4.
For 2H2Cl2
read C2H2Cl2
Page 1132, P-82.2.1, example 6. [modified 3 April 2019]
For 1-phenyl(1,2-13C2)ethanone (PIN)
read 1-phenyl(1,2-13C2)ethan-1-one (PIN)
for (1,2-13C)acetophenone
read (1,2-13C2)acetophenone
Page 1133, P-82.2.1, example 2 on page.
For 2-(13C)methyl-(1-13C)benzene (PIN)
read 1-(13C)methyl(2-13C)benzene (PIN)
for (α,1-13C2)-1,2-xylene
read (α,2-13C2)toluene
delete (α,1-13C2)-o-toluene
Replace structure with:
Page 1133, P-82.2.1, last example.
For 2-(35Cl)chloro-3-[(2H3)methyl](1-2H1)pentane (PIN)
read 2-(35Cl)chloro-3-(2H3)methyl(1-2H1)pentane (PIN)
Page 1134, P-82.2.2.1, example 1. [corrected 7 November 2018]
For N-[(7-131I)iodo-6-iodo-9H-fluoren-2-yl]acetamide (PIN)
read N-[7-(131I)iodo-6-iodo-9H-fluoren-2-yl]acetamide (PIN)
Page 1134, P-82.2.2.1, example 3.
Replace structure with:
Page 1135, P-82.2.2.2, example 1 on this page.
For cyclohexanedi[(14C)-carboxylic acid] (PIN)
read cyclohexane-1,1-di[(14C)carboxylic acid] (PIN)
Page 1136, P-82.2.3, example 4.
For 2,3-di[(2H)hydro]-(2,3-2H2,15N)-1H-indole
read 2,3-di[(2H)hydro](2,3-2H2,15N)-1H-indole
Page 1136, P-82.2.3, last example.
For 6-methyl-2,3-di[(2H2)dihydro](2,3-2H2)napthalen-1-ol (PIN)
read 6-methyl-2,3-di[(2H)hydro]-1,4-dihydro(2,3-2H2)naphthalen-1-ol (PIN)
for
[not 6-methyl-2,3-(2-2H2,3)dihydro-(2,3-2H2)napthalen-1-ol;
read {not 6-methyl-1,4-dihydro-2,3-di[(2H)hydro](2,3-2H2)naphthalen-1-ol;
for not 6-methyl-2-(2H)hydro-3-hydro-(2,3-2H2)napthalen-1-ol;
read not 6-methyl[(2,3-2H2)-1,2,3,4-tetrahydro](2,3-2H2)naphthalen-1-ol;
Page 1136, P-82.2.4, examples 1 and 2.
Move acetic (2H)acid from under example 1 to under example 2
Page 1137, P-82.2.4, example 1 on this page.
For (O-2H,18O)acetic acid (PIN)
read (O-2H,18O)acetic acid (PIN)
Page 1137, P-82.2.4, example 2 on this page. (corrected 27 November 2019)
For (18O-2H)acetic acid (PIN)
read (18O-2H,18O)acetic acid (PIN)
Page 1137, P-82.2.4, example 6 on this page.
For 4-[(2-14C)ethyl]benzoic acid (PIN)
read 4-(2-14C)ethylbenzoic acid (PIN)
Replace structure with:
Page 1137, P-82.2.5, example 1. (modified 1 December 2021)
For (N-2H)acetamide (PIN)
read (N-2H1)acetamide (PIN)
Delete acet(2H)amide
Page 1138, P-82.4.1, example 3.
Replace structure with:
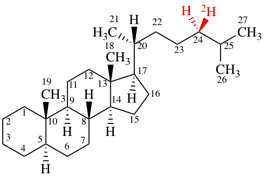
Page 1139, P-82.4.2, example 3.
For 2-(18F) fluoro-2-deoxy-β-D-glucopyranose
read 2-deoxy-2-(18F)fluoro-β-D-glucopyranose [Deleted space from name]
Page 1141, P-82.5.2, example 1 on this page, structural formula. [corrected 19 June 2019]
Replace the structure with:
Page 1141, P-82.5.2, example 3 on this page.
For (2R)-[(1-131I)iodo]-3-iodopropan-2-ol (PIN)
read (2R)-1-(131I)iodo-3-iodopropan-2-ol (PIN)
Page 1141, p-82.6.1.2, example. [modified 8 July 2020]
For (2,3-2H2,15N)pyridine (PIN)
read (2,4-2H2,15N)pyridine (PIN)
Page 1142.
For P-82.6.1.4 (at top of page)
read P-82.6.1.3
Page 1142, P-82.6.1.4, example.
For 2-(79Br)bromo-1-(13C)benzene
read 1-(79Br)bromo(2-13C)benzene (PIN)
Replace structure with:
Page 1142, P-82.6.2, example 3. (modified 1 December 2021)
For (2R)-2(O-2H)hydroxy-3-hydroxy(1-2H)propanal (PIN)
read (2R)-2-(2H)hydroxy-3-hydroxy(1-2H)propanal (PIN)
Delete D-(O-2H)glycer(2H)aldehyde
Page 1143, P-82.6.3.1, example 3.
For L-(α-2H)-phenylalanine (PIN)
read L-(α-2H)phenylalanine (PIN)
Page 1143, P-82.6.3. (corrected 1 December 2021)
For P-82.6.3.2
read P-82.6.3.3
Page 1143, insert new P-82.6.3.2 after P-82.6.3.1 (corrected 1 December 2021)
P-82.6.3.2 When the nuclide is located at a position in a retained name that is not numbered a systematic name that identifies separately the relevant atom is used for the IUPAC preferred name. For general nomenclature an italicized prefixes or Greek letters may be used to denote its position.
benzene(13C)carbonitrile (PIN)
(cyano-13C)benzonitrile
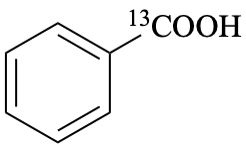
benzene(13C)carboxylic acid (PIN)
(carboxyl-13C)benzoic acid
[benzene[14C]carbonyl]phosphonic acid (PIN)
[phenyl[14C]carbonyl]phosphonic acid
[carbonyl-14C]benzoylphosphonic acid
[phenyl(13C)methyl]hydrazine (PIN)
[(α-13C)benzyl]hydrazine
Page 1143, P-82.6.3.3 (was P-82.6.3.2), example 2.
For 1-propylidene(1-15N)diazene (PIN)
read 1-propylidene(1-15N)hydrazine (PIN)
Page 1144, P-82.6.3.3 (was P-82.6.3.2), example 3.
For 3-[3-ethyl(2-34S)trisulfan-1-yl]propanoic acid (PIN)
read 3-[ethyl(2-34S)trisulfanyl]propanoic acid (PIN)
Page 1144, P-82.6.4, example 5. (modified 27 November 2019)
For (18O-2H)acetic acid (PIN)
read (18O-2H,18O)acetic acid (PIN)
delete (the ‘18O’ is both an isotopic descriptor and a locant).
Page 1144, P-82.6.4, example 6. [corrected 3 April 2019]
For (18O,O-2H)acetic acid
read (O-2H,18O)acetic acid (PIN)
Page 1147, P-83.1.2.2, example 1.
For benzene-1,3,5-tri[13C]carboxylic acid (PIN)
read benzene-1,3,5-tri([13C]carboxylic acid) (PIN)
Page 1147, P-83.1.2.2, example 2. [modified 7 August 2019]
For 3-carboxy-5-[13C]carboxybenzene-1-[18O]carboxylic acid (PIN)
read 3-[13C]carboxy-5-carboxybenzene-1-[18O]carboxylic acid (PIN)
replace the structure with:
Page 1147, P-83.1.2.2, example 3. [corrected 8 July 2020]
For (not benzene-1,3,5-[1,5-13C2,1,3-18O2]tricarboxylic acid)
read (not benzene-1,3,5-[1,3-13C2,1,5-18O2]tricarboxylic acid)
Page 1148, P-83.1.2.2, example 1 on this page.
For 2-carboxy[1-14C]benzene-1-[1-14C]carboxylic acid (PIN)
read 2-carboxy[1-14C]benzene-1-[14C]carboxylic acid (PIN;
Page 1148, P-83.1.2.2, example 3 on this page.
For acetic [14C]acid (PIN;
read [1-14C]acetic acid (PIN)
Page 1148, P-83.1.2.2, example 4 on this page. [modified 19 June 2019]
For 2-[15N]amino-3-hydroxy[2,3-2H2 ,3-14C]propanoic acid
read (2S)-2-[15N]amino-3-hydroxy[2,3-2H2,3-14C]propanoic acid [delete space from name]
Page 1148, P-83.1.2.2, example 5 on this page. (modified 27 November 2019)
For 1-(naphthalene-2-yl)-2-phenyl[2-15N]diazene (PIN)
read 1-(naphthalen-2-yl)-2-phenyl[2-15N]diazene (PIN)
Page 1148, P-83.1.2.2, example 6 on this page. [corrected 19 September 2018]
For O-ethyl 18O-methyl naphthlene-2-yl[18O]phosphonate (PIN)
read O-ethyl 18O-methyl naphthlen-2-yl[18O]phosphonate (PIN)
Page 1148, P-83.1.2.2, example 5 on this page. [corrected 1 December 2021]
For ...(see P-82.6.3.2)
read ...(see P-82.6.3.3)
Page 1148, P-83.1.2.2, example 6 on this page [modified 30 March 2022]
For O-ethyl 18O-methyl naphthalene-2-yl[18O]phosphonate (PIN)
read O-ethyl 18O-methyl (naphthalene-2-yl)[18O]phosphonate (PIN)
Page 1150, P-83.2.1.1, example 4 on this page, the last of the three structures. [corrected 9 January 2019]
Replace the structure with:
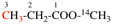
Page 1155, P-84, example 3.
For C[2H2]-CH2-O[2H]
read C[2H3]-CH2-O[2H]
Page 1155, P-84, example 5. [corrected 3 April 2019]
For [2-2H2;2]ethan-1-[18O0;]]ol
read [2-2H2;2]ethan-1-[18O0;1]ol (PIN)
Page 1157-1292, running heading.
For P-9 Specification of Configuration and Confirmation
read P-9 Specification of Configuration and Conformation
Page 1158, P-91.2.1.2.1 (a) (i) , line 4. (modified 27 November 2019)
For ...example, R1R2C= and =C{[CH2}n}C=NOH. The...
read ...example, R1R2C=NOH and HON=C{[CH2]n}C=NOH. The...
Page 1159, P-91.2.1.2.1 (c) (vi) line 4.
For ...a 50:0 mixture.
read ...50:50 mixture. >
Page 1159, P-91.2.1.2.1, last paragraph on this page, line 1. [corrected 28 October 2016]
For In preferred IUPAC names, nonstereogenic units are not specified and in preferred...
read In preferred...
Page 1160, P-91.2.1.2.1, paragraph on this page. [corrected 28 October 2016]
Delete the last sentence.
Page 1160, P-91.3, example 1. [corrected 28 April 2023]
For (R)-bromo(chloro)fluoromethane
read (R)-bromo(chloro)(fluoro)methane
Page 1161, P-91.3, example 2 on this page.
For 1-[(1R)-(1-chloropropyl)]benzene (PIN)
read [(1R)-1-chloropropyl]benzene (PIN)
Page 1161, P-91.3, example 5 on this page.
For (2Z,6S)-6-chlorohept-2-ene
read (2Z,6S)-6-chlorohept-2-ene (PIN)
Page 1164, P-92.1.1 (d), example 2. [corrected 2 January 2019]
For A pseudoasymmetric stereogenic unit denoted by ‘m’ for ‘a > F > ꟻ > d’ ...
read A pseudoasymmetric stereogenic unit denoted by ‘m’ for ‘a > b and F > ꟻ’ ...
Page 1167, P-92.1.2.2, lines 6-8. [corrected 10 April 2019]
For ...considers the torsion angle between two specified (fiducial) groups are attached to the atoms linked by that bond. The sign of the smaller torsion angle between the fiducial groups defines the sense of chirality sense of the helix (see torsion angle, P-94.2).
read ...considers the torsion angle between two reference groups attached to the atoms at each end of a bond or an axis. The sign of the smaller torsion angle between the reference groups defines the chirality sense of the corresponding stereogenic unit (see torsion angle, P-94.2)
Page 1169, P-92.1.3.4 (b)(i), line 2. [corrected 19 September 2018]
For ‘seqTran/seqTrans’
read ‘seqTrans/seqTrans’
Page 1170, P-92.1.4.1, example. [modified 7 August 2019]
For 6-chlorohexane-2,4-diol [PIN; represented by a Fischer projection formula [see Prelog and Helmchen, subsection 3.1, ref. 36 and see also P-102.3.1)]
read 6-chlorohexane-2,4-diol (PIN)
Page 1170, P-92.1.4.1, complete digraph for center 4.
Replace structure with:
Page 1173, P-92.1.4.3, example 1 on this page. [corrected 26 September 2018]
Replace both structures with:
Page 1174, P-92.1.4.4, example on this page.
For 3-[(S)-(cyclopenta-1,3-dien-2-yl)(hydroxy)methyl]cyclopenta-2,4-dien-1-ide (PIN)
read 3-[(S)-(cyclopenta-1,4-dien-1-yl)(hydroxy)methyl]cyclopenta-2,4-dien-1-ide (PIN)
Page 1176, P-92.2.1.1.1, example. [corrected 6 December 2023]
For (R)-bromo(chloro)fluoromethane (PIN)
read (R)-bromo(chloro)(fluoro)methane (PIN)
Page 1177, P-92.2.1.1.1, Example 3.
For (R)-germyl(methoxy)(methylsulfanyl)methyl]silane (PIN)
read [(R)-germyl(methoxy)(methylsulfanyl)methyl]silane (PIN)
Page 1179, P-92.2.1.1.3, Example 1. [modified 2 January 2019]
For (2R,3S,4S,5R,6S)-3-(2-bromoethyl)-1-chloro-2,6,7-trifluoro-5-(2-iodoethyl)heptan-4-ol (PIN)
read (2R,3S,4R,5R,6S)-3-(2-bromoethyl)-1-chloro-2,6,7-trifluoro-5-(2-iodoethyl)heptan-4-ol (PIN)
Replace structure with:

replace structure with:
Page 1181, P-92.2.1.1.3, example 2. [corrected 10 April 2019]
For (2R,3S,4S,5R,6S)-3-(bromoethyl)-1,2,6,7-tetrafluoro-6-(2-iodoethyl)heptan-4-ol (PIN)
read (2R,3S,4S,5R,6S)-3-(bromoethyl)-1,2,6,7-tetrafluoro-5-(2-iodoethyl)heptan-4-ol (PIN)
Page 1183, P-92.2.1.2, Example 3. [corrected 19 September 2018]
For (2R)-2-hydroxypent-3-enenitrile (PIN)
read (2R)-2-hydroxybut-3-enenitrile (PIN)
Page 1183, P-92.2.1.3, Example 1. [corrected 26 September 2018]
Replace both structures with:
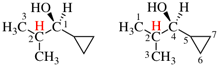
Page 1185, P-92.2.1.3, Example 3.
For (2S)-cyclopropyl(hydroxy)acetaldehyde (PIN)
read (S)-cyclopropyl(hydroxy)acetaldehyde (PIN)
Page 1185, P-92.2.1.3, example 3, explanation, line 4. [corrected 26 June 2019]
For ... branch it is ‘C,H,H’, leading to...
read ... branch it is ‘C,C,H’, leading to...
Page 1185, P-92.2.1.3, Example 4.
For 1-[(1R)-1-chloropropyl]cyclopropane (PIN)
read [(1R)-1-chloropropyl]cyclopropane (PIN)
Page 1186, P-92.2.1.3, Explanation, line 9. [corrected 2 January 2019]
For ... leading to the overall priority of ‘C-2 > C-2 > C-7 >, H’
read ... leading to the overall priority of ‘C-2 > C-6 > C-7 > H’
Page 1186, P-92.2.1.3, Explanation, line 12. [corrected 2 January 2019]
For ... resulting in the priority order ‘C-6 > C-3 > C-8 > H’
read ... resulting in the priority order ‘C-1 > C-3 > C-8 > H’
Page 1187, P-92.2.2, Example 1.
For (1S)-1-(bicyclo[2.2.2}octan-1-yl)-4-cyclopropyl-2,2-bis(2-cyclopropylethyl)butan-1-ol (PIN)
read (1S)-1-(bicyclo[2.2.2]octan-1-yl)-4-cyclopropyl-2,2-bis(2-cyclopropylethyl)butan-1-ol (PIN)
Page 1188, P-92.3, example 1.
For (1R)-1-(1-2H1)-ethan-1-ol
read (1R)-(1-2H1)-ethan-1-ol (PIN)
Page 1189, P-92.3, example 2 and explanation line 2/3. (modified 27 November 2019)
For
For (2R)-1,3-[1-125I]diiodopropan-2-ol (PIN)
read (2S)-1-[125I]iodo-3-iodopropan-2-ol (PIN)
for...is: ‘OH > CH2[125I] > CH2I > H’ and the configuration is ‘R’ for...
read ...is: ‘OH > CH2I > CH2[125I] > H’ and the configuration is ‘S’ for...
Replace the structure with:
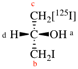
Page 1190, P-92.4.1, example.
Replace the structure with:
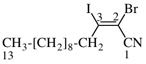
Page 1191, P-92.4.2.1, example 2. [corrected 26 September 2018]
Replace the structure with:
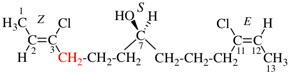
Page 1192, P-92.4.2.2, Example 1. [corrected 2 September 2020]
For 1,3-di[(1E)-ethylidene]cyclobutane
read 1,3-di[(E)-ethylidene]cyclobutane
Page 1193, P-92.4.2.2, Example 2. [modified 2 September 2020]
For (2Z,5Z,7S,8Z,11Z)-9-[(2Z)-but-2-en-1-yl]-5-[(2E)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
read (2Z,5Z,7S,11Z)-5-[(2E)-but-2-en-1-yl]-9-[(2Z)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
replace structures with:
Page 1193, P-92.4.2.2, last example.
For (2Z,5Z,7S,8Z,11Z)-9-[(2Z)-but-2-en-1-yl]-5-[(2E)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
read (2Z,5Z,7S,11Z)-5-[(2E)-but-2-en-1-yl]-9-[(2Z)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
Page 1194, P-92.5.1, Note, line 2. [corrected 10 April 2019]
For ... the stereodescriptor set ‘2R,3S,6R’ in the principal chain ...
read ... the stereodescriptor set ‘2R,4S,6R’ in the principal chain ...
Page 1195, P-92.5.1 Example (cont‘d), line 2. [corrected 10 April 2019]
For ...at ‘C-2’, ‘C-5’, ‘C-6’, ‘C-8’ and ‘C-10’,...
read ...at ‘C-2’, ‘C-3’, ‘C-6’, ‘C-8’ and ‘C-10’,...
Page 1198, P-92.5.2.2, Example 2, Explanation, line 7. [corrected 2 September 2020]
For ... ‘u’. like pairs being ‘RR’ or ‘SS’, unlike pairs...
read ...‘u’, like pairs being ‘RR’ or ‘SS’, unlike pairs...
Page 1199, P-92.5.2.2, Example 3, simplified digraph. [corrected 2 September 2020]
Replace the structure with:

Page 1199, P-92.5.2.2, Example 3, Explanation, lines 2/3. [corrected 2 September 2020]
For ...auxiliary descriptors specified in place of usual stereodescriptors R and S as described...
read ...auxiliary descriptors, R0 and S0, specified in place of usual stereodescriptors R and S as described...
Page 1199, P-92.5.2.2, Example 3, Explanation, line 5. [corrected 2 September 2020]
For ...auxiliary descriptors (R0 and S0). The methodology...
read ...auxiliary descriptors (R0 and S0) illustrated here for C-1. The auxiliary descriptors for every node are determined for this digraph. For example, the auxiliary descriptor for C-6 node is R0 as Cl > C-1 > C-5 > H. Once all necessary auxiliary descriptors are assigned, the methodology...
Page 1200, P-92.5.2.2, example 4.
For (2R,3S,6R,9R,10S)-6-chloro-5-[(1R,2S)-1,2-dihydroxypropoxy]-7-[(1S,2S)-1,2-dihydroxypropoxy]- 4,8-dioxa-5,7-diazaundecane-2,3,9,10-tetrol (PIN; [in the principal chain, both pairs of stereodescriptors are unlike; thus, stereodescriptor R is assigned to the lowest possible position, ‘2’)
read (2R,3S,6R,9R,10S)-6-chloro-5-[(2R,3S)-2,3-dihydroxybutyl]-7-[(2S,3S)-2,3-dihydroxybutyl]-4,8-dioxa-5,7-diazaundecane-2,3,9,10-tetrol
(PIN; in the principal chain, named by skeletal replacement nomenclature, both pairs of stereodescriptors are unlike so the stereodescriptor R is assigned the lowest locant, ‘2’). [delete space in name and change explanation]
Page 1201, P-92.5.2.1, example 5. [modified 10 April 2019]
For (2R,3R,5R,7R,8R)-4.4-bis[(2S,3R)-3-chlorobutan-2-yl]-6,6-bis[(2S,4S)-3-chlorobutan-2-yl)]-2,8-dichloro-3,7-dimethylnonan-5-ol (PIN; ...
read (2R,3R,5R,7R,8R)-2,8-dichloro-4,4-bis[(2S,3R)-3-chlorobutan-2-yl]-6,6-bis[(2S,3S)-3-chlorobutan-2-yl]-3,7-dimethylnonan-5-ol (PIN; ...
delete CH4 from structure
Page 1202, P-92.5.2.2, example 5 (continued).
Replace digraph for C-5 with
Page 1203, P-92.5.2.2, Example 5, paragraph 3, line 2 and 3.
For ...pair S,8 and S,11 (like pair) over the pair S,2 and S,17 (unlike pair)...
read ...pair S,8 and S,11 (like pair) over the pair S,2 and S,17 (unlike pair)...
Page 1204, P-92.5.2.2, Example 6.. [corrected 12 May 2021]
For (2R)-2-{bis[(1R)-1-hydroxyethyl]amino}{[(1R)-1-hydroxyethyl]-2-[(1S)-1-hydroxyethyl]amino}acetic acid (PIN)
read (R)-{bis[(1R)-1-hydroxyethyl]amino}{[(1R)-1-hydroxyethyl][(1S)-1-hydroxyethyl]amino}acetic acid (PIN)
Page 1204, P-92.5.3, example. [corrected 10 October 2018]
Replace the structure with:
Page 1205, P-92.6, title. [corrected 2 September 2020]
For ...and ‘seqCis’ precedes ‘seqTrans’.
read ...and ‘seqCis’ precedes ‘seqTrans’, and similarly for auxiliary descriptors.
Page 1206, P-92.6, Example 2.
Replace structure with:
Page 1206, P-92.6, example 3.
For (3R,4m,5S)-4-(2-bromoethenylidene)heptane-3,5-dithiol (PIN)
read (3R,4m,5S)-4-(2-bromoethen-1-ylidene)heptane-3,5-dithiol (PIN)
for (3R,4ra,5S)-4-(2-bromoethenylidene)heptane-3,5-dithiol
read (3R,4ra,5S)-4-(2-bromoethen-1-ylidene)heptane-3,5-dithiol
Page 1207, P-92.6, example 4 (1 on this page).
For (11Sp)-12-[(1R)-1-bromoethyl]-16-[(1S)-1-bromoethyl]-1,4(1,4)-dibenzenacyclohexaphane (PIN)
read (11sp)-12-[(1R)-1-bromoethyl]-16-[(1S)-1-bromoethyl]-1,4(1,4)-dibenzenacyclohexaphane
Page 1207, P-92.6, example 5 (2 on this page).
Replace structures with:
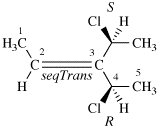
Page 1208, P-92.6, Example 6. [modified 11 November 2020]
For (1s)-1-{[(1R,2R)-1,2-dichloropropyl][(1S,2R)-1,2-dichloropropyl]amino}-1-{[(1R,2S)-1,2-dichloropropyl][(1S,2S)-1,2-dichloropropyl]amino}methan-1-ol
read (s)-{[(1R,2R)-1,2-dichloropropyl][(1S,2R)-1,2-dichloropropyl]amino}{[(1R,2S)-1,2-dichloropropyl][(1S,2S)-1,2-dichloropropyl]amino}methanol
replace the structure with:
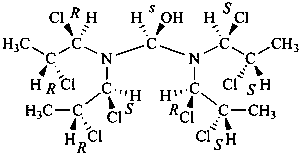
Page 1209, P-92.6, Example 6, last paragraph on this page line 6. [corrected 2 September 2020]
For ...ranking node 8 is 'S in 'branch B';...
read ..ranking node 14 is 'S in 'branch B';...
Page 1209, P-92.6, Example 6, Table at bottom of page, Branch B. [modified 2 September 2020]
For 8-'S' (first difference encountered)
read 14-'S' (first difference encountered)
for 14-'R'
read 8-'S'
Page 1209, P-92.6, example 6 (cont’d), Branch B, last line. [corrected 2 January 2019]
For 14-‘R’
read 14-‘S’
Page 1211, P-93.1.2.1, Example 1.
For cis-2-chlorocyclopentane-1-carboxylic acid (see P-93.5.1.2 for use of the non-CIP stereodescriptor ‘cis’)
read trans-2-chlorocyclopentane-1-carboxylic acid (see P-93.5.1.2 for use of the non-CIP stereodescriptor ‘trans’)
Page 1212, P-93.1.2.2, example.
For (1R*,3R*,5S*)-1-[(2S)-butan-2-yloxy]-3-chloro-5-nitrocyclohexane (PIN)
read (1R*,3R*,5S*)-1-{[(2S)-butan-2-yl]oxy}-3-chloro-5-nitrocyclohexane (PIN)
Page 1213, P-93.1.4, example.
For 2-ambo-(2R)-2,5,7,8-tetramethyl-2[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol (PIN)
read 2-ambo-(2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol (PIN)
Page 1214, P-93.2.1, lines 7/8. [corrected 18 September 2019]
For ...atom is B, Al, Ga, In, Tl, S, Se, Te, N, P, As, Sb, Bi is written...
read ...atom is B, S, Se, Te, N, P, As, Sb, Bi is written...
Page 1214, P-93.2.2, example 1.. [modified 12 May 2021]
For (R)-[N-benzyl-N-methyl-N-(prop-2-en-1-yl)benzenaminium] bromide (PIN)
read (R)-N-benzyl-N-methyl-N-(prop-2-en-1-yl)benzenaminium bromide
for (R)-[benzyl(methyl)phenyl(prop-2-en-1-yl)azanium] bromide
read (R)-benzyl(methyl)(phenyl)(prop-2-en-1-yl)azanium bromide
for (R)-[benzyl(methyl)phenyl(prop-2-en-1-yl)ammonium] bromide
read (R)-benzyl(methyl)(phenyl)(prop-2-en-1-yl)ammonium bromide
add (R)-N-benzyl-N-methyl-N-(prop-2-en-1-yl)anilinium bromide (PIN)
Page 1214, P-93.2.2, example 2.
For (2S)-(4-hydroxyphenyl)-2-phenyl-1,2,3,4-tetrahydrophosphinolin-2-ium bromide (PIN)
read (2S)-2-(4-hydroxyphenyl)-2-phenyl-1,2,3,4-tetrahydroisophosphinolin-2-ium bromide (PIN)
Page 1215, P-93.2.3, example 1.. [corrected 12 May 2021]
For (S)-N-ethyl-N-methylbenzenaminiumyloxidanide
read [(S)-N-ethyl-N-methylbenzenaminiumyl]oxidanide
Page 1215, P-93.2.3, example 1. [modified 21 April 2021]
For (S)-N-ethyl-N-methylbenzenamine N-oxide (PIN;
read (S)-N-ethyl-N-methylaniline N-oxide (PIN;
for (S)-(N-ethyl-N-methylbenzeneaminiumyl)oxidanide
read (S)-[ethyl(methyl)phenylazaniumyl]oxidanide
Page 1215, P-93.2.3, example 2. . [modified 12 May 2021]
For (S)-[methyl(phenyl)propyl-λ5-phosphanone] (PIN;
read (S)-methyl(phenyl)(propyl)-λ5-phosphanone (PIN;
for (S)-methyl(phenyl)propylphosphane oxide
read (S)-methyl(phenyl)(propyl)phosphane oxide
Page 1216, P-93.2.4, example 3 on this page. [modified 30 September 2020]
For dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
read dihydrogen (S)-[O-methyl (17O1,18O1)phosphate] (PIN)
Page 1216, P-93.2.4, example 3 on this page. [modified 3 March 2021]
Replace the structure with:
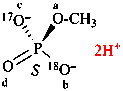
Page 1216, P-93.2.4, last example.
For dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
read dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
Page 1217, P-93.2.5, example 1 on this page. [modified 21 August 2019]
For 1-methyl-4-{(R)-phenyl[(18O1)methanesulfonyl}benzene (PIN)
read 1-methyl-4-[(R)-phenyl(18O1)methanesulfonyl]benzene (PIN)
replace structure with:
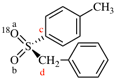
Page 1217, P-93.2.5, example 2 on this page. [modified 30 September 2020]
For N,N,N-tributylbutanaminium (S)-[O-phenyl (17O,18O)sulfate] (PIN)
read N,N,N-tributylbutan-1-aminium (S)-[O-phenyl (17O1,18O1)sulfate] (PIN)
for tetrabutylammonium (S)-[O-phenyl (17O,18O)sulfate]
read tetrabutylammonium (S)-[O-phenyl (17O1,18O1)sulfate]
for tetrabutylazanium (S)-[O-phenyl (17O,18O)sulfate]
read tetrabutylazanium (S)-[O-phenyl (17O1,18O1)sulfate];
replace structure with:
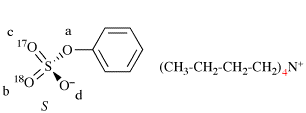
Page 1224, P-93.3.3.6, example 2.
For (SPY-5-21′)-2-phenyl-2λ5,2′-spirobi[1,3,2-benzodioxaphosphole] (PIN)
read (SPY-5-21′)-2-phenyl-2H-2λ5,2′-spirobi[[1,3,2]benzodioxaphosphole] (PIN)
Page 1225, P-93.3.3.6, example 3.
For (SPY-5-21′)-2-phenyl-2,2′-spirobi[naphtho[2,3-d][1,3,2]dioxasilol]-2-uide (PIN)
read (SPY-5-21′)-2-phenyl-2H-2,2′-spirobi[naphtho[2,3-d][1,3,2]dioxasilol]-2-uide (PIN)
Page 1227, P-93.3.4.1, lines 5-7.
Delete the sentence:
As no locants are present, the name following the stereodescriptor is placed in parentheses, or bracket, according to the required nesting order.
Page 1227, P-93.3.4.1, example 1.
For (S)-[(methanesulfinyl)ethane]
read (S)-(methanesulfinyl)ethane (PIN)
Page 1227, P-93.3.4.1, example 2.
For (S)-[(methanesulfinyl)benzene] (PIN)
read (S)-(methanesulfinyl)benzene (PIN)
Page 1227, P-93.3.4.1, example 3.
For ethyl (R)-(4-nitrobenzene-1-sulfinate) (PIN)
read ethyl (R)-4-nitrobenzene-1-sulfinate (PIN)
Page 1227, P-93.3.4.1, example 4.. [modified 12 May 2021]
For (R)-[methyl(phenyl)propylphosphane] (PIN)
read (R)-methyl(phenyl)(propyl)phosphane (PIN)
Page 1228, P-93.3.4.2.1, example 1.
For (TPBY-5-12-C)-2-phenoxy-2λ5-spiro[1,3,2-dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
read (TPBY-5-12-C)-2-phenoxy-2H-2λ5-spiro[[1,3,2]dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
Page 1228, P-93.3.4.2.1, Example 1, Explanation, the right-hand structure. [corrected 10 October 2018]
Replace structure with:
Page 1229, P-93.3.4.2.1, example 2.
For (SS-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4-spirobi[2,1-benzoxathiole] (PIN)
read (SS-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4,1′-spirobi[[2,1]benzoxathiole] (PIN)
for {not (TBPY-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4-spirobi[2,1-benzoxathiole]}
read {not (TBPY-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4,1′-spirobi[[2,1]benzoxathiole]}
Page 1230, P-93.3.4.2.1, example 4.
For (OC-6-22′-A)-1,1-difluoro-3,3,3′,3′-tetrakis(trifluoromethyl)-3H,3′H-1λ6-spirobi[2,1-benzoxathiole] (PIN)
read
(OC-6-22′-A)-1,1-difluoro-3,3,3′,3′-tetrakis(trifluoromethyl)-1,3′-dihydro-3H-1λ6,1′-spirobi[[2,1]benzoxathiole] (PIN)
Page 1231, P-93.3.4.2.2, example, Explanation. [corrected 24 October 2018]
Replace left structure with:
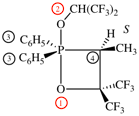
Page 1231, P-93.4.
Delete P-93.4.3 Specification of configuration of cumulenes
delete P-93.4.4 Specification of configuration of unsaturated compounds
Page 1232, P-93.4.1.1, example 2 on this page.
For (1R)-(1-2H)ethan-1-ol (PIN)
read (1R)-(1-2H1)ethan-1-ol (PIN)
Page 1234, P-93.4.1.2, last example.
For (2R,3R)-2,3-dichloro-(2-2H)butan-2-ol (PIN)
read (2R,3R)-2,3-dichloro-(2-2H)butane (PIN)
replace diagram with:
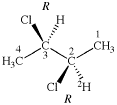
Page 1236, P-93.4.2.1.1, example 1 on this page.
For (2Z)-2,3-dibromoprop-2-enenitrile (PIN)
read (2Z)-2,3-dibromo-3-iodoprop-2-enenitrile (PIN)
for (2Z)-2,3-dibromoacrylonitrile
read (2Z)-2,3-dibromo-3-iodoacrylonitrile
Page 1236, P-93.4.2.1.1, example 3 on this page.
Replace structure with:
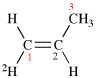
Page 1236, P-93.4.2.1.1, last example.
For (1E)-1,1′-[1-(4-chlorophenyl)ethene-1,2-diyl]dibenzene (PIN)...
read 1,1′-[(1E)-1-(4-chlorophenyl)ethene-1,2-diyl]dibenzene (PIN)...
Page 1236, P-93.4.2.1.2, 3rd paragraph, line 3/5. [corrected 26 June 2019]
For ... The stereodescriptors, ‘cis’ and ‘trans’, placed in parentheses, are cited at the front of the name, preceded by the appropriate locant followed by a hyphen.
read ... The stereodescriptors, ‘cis’ and ‘trans’, are cited at the front of the name, preceded by the appropriate locant, if necessary, followed by a hyphen.
Page 1236, P-93.4.2.1.2, example 1. [corrected 10 April 2019]
For (2-cis,4-trans)-octa-2,4-diene
read 2-cis,4-trans-octa-2,4-diene
Page 1237, P-93.4.2.1.2, example 1 on this page. [corrected 10 April 2019]
For (2-trans,4-cis)-hexa-2,4-dienoic acid
read 2-trans,4-cis-hexa-2,4-dienoic acid
Page 1237, P-93.4.2.1.2, example 3 on this page. [corrected 10 April 2019]
For (3-cis,5-trans)-octa-3,5-diene
read 3-cis,5-trans-octa-3,5-diene
Page 1237, P-93.4.2.1.3, example 1.
For (Z)-(diphenyldiazene) (PIN)
read (Z)-diphenyldiazene (PIN)
for (not trans-diphenyldiazene)
read (not cis-diphenyldiazene)
Page 1238, P-93.4.2.1.3, example 1 on this page. [modified 2 September 2020]
For (Z)-{N-[(4-chlorophenyl)(phenyl)methylidene]hydroxylamine} (PIN)
read (Z)-N-[(4-chlorophenyl)(phenyl)methylidene]hydroxylamine
for (Z)-[(4-chlorophenyl)phenylmethanone oxime]
read (Z)-(4-chlorophenyl)(phenyl)methanone oxime
add (Z)-N-hydroxy(4-chlorophenyl)(phenyl)methanimine (PIN)
Page 1238, P-93.4.2.1.3, example 2 on this page. [corrected 24 July 2019]
For (2E,3Z)-pentane-2,3-diylidenebis(hydroxylamine) (PIN)
read (2E,3Z)-(pentane-2,3-diylidene)bis(hydroxylamine)
add (2E,3Z)-N2,N3-dihydroxypentane-2,3-diimine (PIN)
Page 1239, P-93.4.2.3, example 2. [corrected 10 April 2019]
For (2-trans,6-trans)-octa-2,3,4,6-tetraene
read 2-trans,6-trans-octa-2,3,4,6-tetraene
Page 1240, P-93.4.2.4, example 1 on this page. [corrected 10 April 2019]
For (5S,6-cis,8-trans,10-trans,12R,14-cis)-5,12-dihtydroxyicosa-6,8,10,14-tetraenoic acid
read 6-cis,8-trans,10-trans,14-cis-(5S,12R)-5,12-dihtydroxyicosa-6,8,10,14-tetraenoic acid
Page 1240, P-93.4.2.4, example 2 on this page. [corrected 10 April 2019]
For (2R)-3-hydroxypropane-1,2-diyl di[(3-trans,5-trans)-hepta-3,5-dienoate]
read (2R)-3-hydroxypropane-1,2-diyl di(3-trans,5-trans-hepta-3,5-dienoate)
Page 1241, P-93.5.1.1.1, example 1. [corrected 10 October 2018]
Replace the structure with:
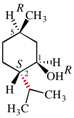
Page 1241, P-93.5.1.1.1, example 2.
Replace structure with:
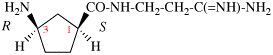
Page 1242, P-93.5.1.1.1, example 3 on this page
Replace structure with:
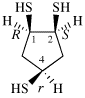
Page 1242, P-93.5.1.1.2 (a), example 1.
Add cis-cyclohexane-1,4-diol
Page 1242, P-93.5.1.1.2 (a), example 2.
For 1r,4r)-cyclohexane-1,4-diol (PIN)
read (1r,4r)-cyclohexane-1,4-diol (PIN)
add trans-cyclohexane-1,4-diol
Page 1243, P-93.5.1.1.2 (a), example 1 on this page.
Add trans-4-chloro-4-methylcyclohexan-1-ol
Page 1243, P-93.5.1.1.2 (a), example 2 on this page.
Add bis(trans-4-methylcyclohexyl)phosphane
Page 1243, P-93.5.1.1.2 (a), example 3 on this page. [modified 26 June 2019]
For bis[(1r,4r)-4-methylcyclohexyl]-(1s,4s)-4-methylcyclohexylphosphane (PIN)
read bis[(1r,4r)-4-methylcyclohexyl][(1s,4s)-4-methylcyclohexyl]phosphane (PIN)
add (cis-4-methylcyclohexyl)bis(trans-4-methylcyclohexyl)phosphane
Page 1243, P-93.5.1.1.2 (b), line 8.
For (1R,2R3S,4R,5S,6S)-1,2,3,4,5,6-hexachlorocyclohexane
read (1R,2R,3S,4R,5S,6S)-1,2,3,4,5,6-hexachlorocyclohexane
Page 1243, P-93.5.1.1.2 (b), end of paragraph. [corrected 31 May 2023]
Add The numbering for these examples is decided by application of criterion P-14.4 (j).
Page 1243, P-93.5.1.1.2 (b), example 1.
For 1 1R,2R,3S,4S,5R,6S)
read 1 (1R,2R,3S,4R,5S,6S)
replace structure with:
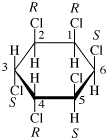
Page 1244, P-93.5.1.1.2 (b), first example on this page, 3. [modified 31 May 2023]
For 3 (1R,2s,3S,4R,5s,6S)
read 3 (1R,2r,3S,4R,5s,6S)
Note R > r > S > s [see P-14.4 (j)]
replace the structure with:
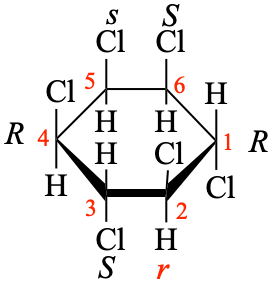
Page 1244, P-93.5.1.1.2 (b), second example on this page, 4, add after the name. [corrected 31 May 2023]
Add Note R > r > S > s [see P-14.4 (j)]
Page 1245, P-93.5.1.2, last name on this page, line 2.
For ... 1,1,2,2-tetracarbonitrile (I) (PIN; ...
read ... 1,1,2,2-tetracarbonitrile (PIN; ...
Page 1246, P-93.5.1.3.1, lines 3-5. [corrected 3 September 2018]
For ...by adding ‘r’ (the reference ligand) followed by a hyphen and the locant of the lowest numbered of these ligands, and ‘c’ for ‘cis’ and ‘t’ for ‘trans’ (as required) followed by a hyphen and the locant of another ligand,...
read ...by adding ‘r’ (the reference ligand) after the locant of the lowest numbered of these ligands, and ‘c’ for ‘cis’ and ‘t’ for ‘trans’ (as required) after the locant of another ligand,...
Page 1246, P-93.5.1.3.1, 2nd paragraph, lines 1-3. (corrected 27 November 2019)
For ...reference substituent) followed by a hyphen and the locant of the lowest numbered of these substituents and ‘c’ (for cis) and ‘t’ (for trans) (as appropriate) followed by a hyphen and the locant...
read ...reference substituent) after the locant of the lowest numbered of these substituents and ‘c’ (for cis), ‘t’ (for trans) (as appropriate) and the locant...
Page 1246, P-93.5.1.3.1, Example 1. [Corrected 3 September 2018]
For 1-r,2-c,4-c-tribromocyclohexane
read 1r,2c,4c-tribromocyclohexane
replace structure with:
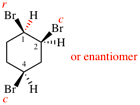
Page 1246, P-93.5.1.3.1, Example 2. [Corrected 3 September 2018]
For 2-r,3-t,4-c-trichlorocyclopentane-1,1-dicarboxylic acid
read 2r,3t,4c-trichlorocyclopentane-1,1-dicarboxylic acid
replace structure with:
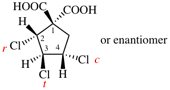
Page 1247, P-93.5.1.3.2, Example 1. [Corrected 3 September 2018]
For 1,2-t-dichlorocyclopentane-1-r-carboxylic acid
read 1,2t-dichlorocyclopentane-1r-carboxylic acid
replace structure with:
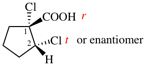
Page 1247, P-93.5.1.3.2, Example 2. [Corrected 3 September 2018]
For 1-r-bromo-1-chloro-3-t-ethyl-3-methylcyclohexane
read 1r-bromo-1-chloro-3t-ethyl-3-methylcyclohexane
replace structure with:
Page 1247, P-93.5.1.3.2, Example 3. [Corrected 3 September 2018]
For 1-r,2-c,3-c,4-t,5-t,6-t-hexachlorocyclohexane
read 1r,2c,3c,4t,5t,6t-hexachlorocyclohexane
replace structure (showing positions of r, c and t) with:
Page 1248, P-93.5.1.4.1, example 5.
For (1-cis,3-trans)-cyclodeca-1,3-diene
read 1-cis,3-trans-cyclodeca-1,3-diene
Page 1248, P-93.5.1.4.1, example 6.
For (1R-trans,4S,7-cis)-4-(propan-2-yl)cyclodeca-2,7-dien-1-ol
read 2-trans,7-cis-(1R,4S)-4-(propan-2-yl)cyclodeca-2,7-dien-1-ol
Page 1249, P-93.5.1.4.2.1 (1) (b), line 3. [corrected 2 September 2020]
For ...substituent group; without a locant; the name of...
read ...substituent group; the name of...
Page 1249, P-93.5.1.4.2.1 (1), example 1. [modified 2 September 2020]
For 1-[(1E)-butan-2-ylidene] 1H-indene
read 1-[(2E)-butan-2-ylidene]-1H-indene
Page 1250, P-93.5.1.4.2.1 (2), Example 2. [corrected 10 April 2019]
For 1,4-di[(1Z)-ethylidene]cyclohexane
read 1,4-di[(Z)-ethylidene]cyclohexane
Page 1250, P-93.5.1.4.2.1 (2), example 3.
For [1(1′)E,3E′,3E)]3,3′-diethylidene-1,1′-bis(cyclobutylidene) (PIN)
read [1(1′)E,3E′,3E]-3,3′-diethylidene-1,1′-bi(cyclobutylidene) (PIN)
for trans-3,3′-diethylidene-1,1′-bis(cyclobutylidene)
read trans-3,3′-diethylidene-1,1′-bi(cyclobutylidene)
for 3,3′-di[(E)-ethylidene]-1,1′-bis(cyclobutylidene)
read 3,3′-di[(E)-ethylidene]-1,1′-bi(cyclobutylidene)
Page 1251, P-93.5.1.4.2.1, example 3, simplified digraph for 5 and 8. [corrected 2 January 2019]
Replace structure with:
Page 1251, P-93.5.1.4.2.2, example 1. [modified 24 March 2021]
For N-[(1seqCis,4R)-4-methylcyclohexylidene]hydroxylamine (PIN)
read [(1seqCis,4R)-4-methylcyclohexylidene]hydroxylamine
add (1seqCis,4R)-N-hydroxy-4-methylcyclohexan-1-imine (PIN)
Page 1252, P-93.5.1.4.2.2, Example 2. [corrected 9 January 2019]
For (3P)-1-(bromomethylidene)-3-propylcyclobutane
read (P)-1-(bromomethylidene)-3-propylcyclobutane
Add (Sa)-1-(bromomethylidene)-3-propylcyclobutane
Page 1252, P-93.5.1.4.2.2, example 3.
For (1S,4p)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
read (1s,4p)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
for (1S,4sa)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
read (1s,4sa)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
replace digraph for C-1 with:
Page 1253, P-93.5.1.4.2.2, example 4 (cont’d).
Replace digraph for C-1=C-1′ with:
digraph for C-4 with:
Page 1253, P-93.5.1.4.2.2, Example 4, Explanation, lines 8-10. [corrected 10 April 2019]
For ... the double bonds ‘C-1–C-1′’; priority being assigned to Sequence Rule 5, the ligand ‘C-4’ has priority over ligand ‘C-3’, ...
read ... the double bonds ‘C-1=C-1′’; priority being assigned to Sequence Rule 5, the ligand ‘C-5’ has priority over ligand ‘C-3’, ...
Page 1254, P-93.5.2.1, example 1. [corrected 2 January 2019]
Replace the structure with:
Page 1255, P-93.5.2.1, last example.
For (1S,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
read (1R,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
replace structure with:
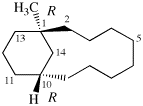
Page 1255, P-93.5.2.2, lines 1/2. [corrected 2 September 2020]
For ...stereodescriptors 'R' and 'S' and prefixes...
read ...stereodescriptors 'R' and 'S' and prefixes...
Page 1256, P-93.5.2.2.1, example 1 on this page. [modified 2 September 2020]
For (2-endo)-bromo-(7-anti)-fluorobicyclo[2.2.1]heptane
read 2-endo-bromo-7-anti-fluorobicyclo[2.2.1]heptane
for rel-(2R,7S)-2-bromo-7-fluorobicyclo[2.2.1]heptane (PIN)
read rel-(1R,2S,4R,7R)-2-bromo-7-fluorobicyclo[2.2.1]heptane (PIN)
replace structure with:
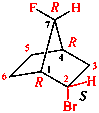
Page 1256, P-93.5.2.2.1, example 2.
For (5-exo)-bromo-(5-endo,7-anti)-dimethylbicyclo[2.2.1]hept-2-ene
read (±)-5-exo-bromo-5-endo,7-anti-dimethylbicyclo[2.2.1]hept-2-ene
for (5S,7R)-dimethylbicyclo[2.2.1]hept-2-ene (PIN)
read rac-(1R,4S,5S,7R)-5-bromo-5,7-dimethylbicyclo[2.2.1]hept-2-ene (PIN)
replace structure with:
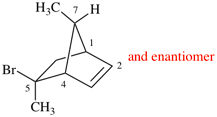
Page 1256, P-93.5.2.2.1, example 3.
For syn-8-methylbicyclo[3.2.1]octane
read 8-syn-methylbicyclo[3.2.1]octane
Page 1257, P-93.5.2.2.1, example on this page. [corrected 13 January 2021]
For 8-methyl-8-aza-bicyclo[3.2.1]heptan-3-endo-yl (2S)-3-hydroxy-2-phenylpropanoate
read 8-methyl-8-aza-bicyclo[3.2.1]octan-3-endo-yl (2S)-3-hydroxy-2-phenylpropanoate
Page 1257, P-93.5.2.2.2, example.
For rel-(1R,10S)-1-methylbicyclo[8.3.1]tetradecane (PIN)
read rel-(1R,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
for (1R*,10S*)-1-methylbicyclo[8.3.1]tetradecane
read (1R*,10R*)-1-methylbicyclo[8.3.1]tetradecane
replace structure with:
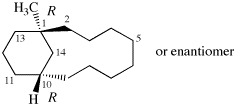
Page 1258, P-93.5.2.3, example 1 on this page. [corrected 2 January 2019]
Add (1S,4E,12R)-bicyclo[10.2.2]hexadeca-4,13-diene >
Page 1258, P-93.5.2.3, example 3 on this page (second cumulene). [corrected 31 October 2018]
For [2S,26R,1(44)E]-bicyclo[24.20.1]heptatetraconta-1(46),44,45-trien-2-ol (PIN)
read [1(44)E,2S,26R]-bicyclo[24.20.1]heptatetraconta-1(46),44,45-trien-2-ol (PIN)
Page 1258, P-93.5.2.3, example 4 on this page (third cumulene).
For (1P)-bicyclo[23.19.1]pentatetraconta-1,2-diene (PIN)
read (1P,25R)-bicyclo[23.19.1]pentatetraconta-1,2-diene (PIN)
Page 1258, P-93.5.2.3, last example on this page. [modified 7 November 2018]
For [1(43)P,2R,25R)-bicyclo[23.19.1]pentatetraconta-1(44),43-diene-2-carboxylic acid (PIN)
read [1(43)P,2R,25R]-bicyclo[23.19.1]pentatetraconta-1(44),43-diene-2-carboxylic acid (PIN)
replace structure with:
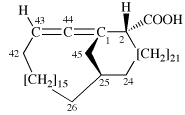
Page 1259, P-93.5.3.1, example 1.
For (1R)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1R)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
Page 1260, P-93.5.3.1, example 4, simplified digraph. [corrected 26 June 2019]
Replace the structure with:
Page 1261, P-93.5.3.1, Example 5. For ...[5a,9a](epiminomethano)cyclopenta[f]indolizin-10′-one (PIN)
read ...[5a,9a](azanomethano)cyclopenta[f]indolizin-10′-one (PIN)
replace structure with:
Page 1261, P-93.5.3.2, Example 1, Explanation, line 4. [corrected 10 April 2019]
For ... atom numbered 6; they are ranked ‘a’ and ‘b’, respectively. ...
read ... atom numbered 6; they are ranked ‘a’ and ‘a′’, respectively. ...
Page 1262, P-93.5.3.2, example 3. [corrected 24 October 2018]
Replace structure with:
Page 1263, P-93.5.3.2, Example 5. [modified 2 September 2020]
For (3R,4s,7S,10s)-tetraoxatetraspiro[2.0.24.0.27.0.210.03]dodecane (PIN)
read (3R,4r,7S,10s)-1,5,8,11-tetraoxatetraspiro[2.0.24.0.27.0.210.03]dodecane (PIN)
replace structure with:
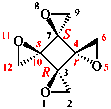
Page 1263, P-93.5.3.2, Example 5, explanation, part (b) [formerly (c)]. [corrected 2 September 2020]
For ...using Ssequence Rule 5, ‘R’ precedes ‘S’, as determined in (b), in accordance ...
read ...using Sequence Rule 5, ‘R0’ precedes ‘S0’, in accordance ...
Page 1263, P-93.5.3.3 example 2. [corrected 2 January 2019]
Add (6Z)-spiro[4.7]dodeca-2,6-diene
Page 1264, P-93.5.3.4, example 1 on this page. [corrected 10 April 2019]
For (TPBY-5-12-C)-2-phenoxy-2λ5-spiro[1,3,2-dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
read (TPBY-5-12-C)-2-phenoxy-2H-2λ5-spiro[[1,3,2]dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
Replace the right-hand structure with:

Page 1264, P-93.5.3.4, example 2.
For (OC-6-22′-A)-4,4′di-tert-butyl-6,6,6′,6′-tetramethyl-2H,2′H,6H,6′H-8λ6,8′-spirobi[[1,2]oxathiolo[4,3,2-hi][2,1]benzooxathiole]-2,2′-dione
read (OC-6-22′-A)-4,4′-di-tert-butyl-6,6,6′,6′-tetramethyl-2H,2′H,6H,6′H-8λ6,8′-spirobi[[1,2]oxathiolo[4,3,2-hi][2,1]benzooxathiole]-2,2′-dione
Page 1264, P-93.5.3.5, Example 1. (corrected 4 December 2019)
Replace the structures with:
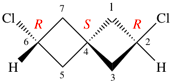
Page 1265, P-93.5.3.5, Example 1, first digraph. (modified 4 December 2019)
Replace the digraph with:
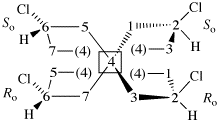
replace digraph 2 with:
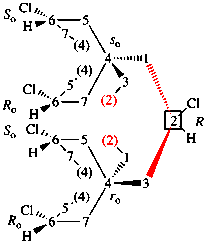
Add This is an example of an ‘Xaabb’ system, see P-93.5.3.2.
Page 1265, P-93.5.3.5, example 1, second digraph. [modified 2 September 2020]
Replace with:

Page 1265, P-93.5.3.5, Example 2. [modified 10 April 2019]
For (5s,11s)-1,12-dioxatrispiro[4.2.2.411.28..25]nonadecane (PIN) (spiro atoms 5 and 11 are pseudoasymmetric centers and spiro atom 8 is nonstereogenic).
read (5S,8R,11S)-1,12-dioxatrispiro[4.2.2.411.28.25]nonadecane (PIN)
for
(5M )-1,12-dioxatrispiro[4.2.2.411.28..25]nonadecane
read (5M)-1,12-dioxatrispiro[4.2.2.411.28.25]nonadecane
[Delete space from name and dot after 8 in both names]
Page 1266, P-93.5.4.1, example 1.
For (5S,11S)-6H,12H-2,8-dimethyl-5,11-methanodibenzo[b,f][1,5]diazocine (PIN)
read (5S,11S)-2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (PIN)
Page 1267, P-93.5.4.1, example 1 on this page. [corrected 2 January 2019]
For(1R,2r,3S,3aR,4S,7R,7aS)-1,2,3,4,5,6,7,8,8-nonachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano-1H- indene (PIN)
read(1R,2r,3S,3aR,4S,7R,7aS)-1,2,3,4,5,6,7,8,8-nonachloro-2,3,3a,4,7,7a-hexahydro-1H-4,7-methanoindene (PIN) [remove space from name]
Page 1267, P-93.5.4.1, example 2 on this page. [modified 5 August 2017)
For (10aM)-1,11-dinitro-5,7-dihydrodibenzo[a,c][7]annulen-6-one (PIN)
read (11aM)-1,11-dinitro-5,7-dihydro-6H-dibenzo[a,c][7]annulen-6-one (PIN)
for (10aRa)-1,11-dinitro-5,7-dihydrodibenzo[a,c][7]annulen-6-one
read (11aRa)-1,11-dinitro-5,7-dihydro-6H-dibenzo[a,c][7]annulen-6-one
replace structure with:
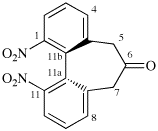
Page 1268, P-93.5.4.1, example 1 on this page.
For (4as,8as)-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene (PIN)
read (4as,8as)-decahydronaphthalene (PIN)
Page 1268, P-93.5.4.1, example 2 on this page.
For (4ar,8ar)-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene (PIN)
read (4ar,8ar)-decahydronaphthalene (PIN)
Page 1269, P-93.5.4.2, example 1.. [corrected 12 May 2021]
For [cis-cisoid-cis-tetrahydroanthracene]
read cis-cisoid-cis-tetrahydroanthracene
Page 1269, P-93.5.4.2, example 2, right version. [corrected 2 January 2019]
Replace the right-hand structure with:
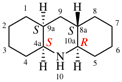
Page 1269, P-93.5.4.2, example 2.. [corrected 12 May 2021]
For [cis-4a-cisoid-4a,10a-trans-10a-tetradecahydroacridine]
read cis-4a-cisoid-4a,10a-trans-10a-tetradecahydroacridine
Page 1270, P-93.5.5.1, example 1.
Replace the structure with:
Page 1270, P-93.5.5.1, example 2. [corrected 28 May 2024]
For (14M)-15-bromo-2,13-dioxa-1(1,4)-benzenacyclotridecaphane-12-carboxylic acid (PIN)
read (14M)-15-bromo-2,13-dioxa-1(1,4)-benzenacyclotridecaphane-12-carboxylic acid
add (1M)-17-bromo-2,13-dioxabicyclo[12.2.2]octadeca-1(16),14,17-triene-15-carboxylic acid (PIN)
Page 1270, P-93.5.5.1, example 3.
Replace the structure with:
Page 1271, P-93.5.5.2, example. [corrected 12 September 2018]
For (2s,4s,6s,8s)-2,4,6,8-tetranonyl-1,3,5,7(1,3)-tetrabenzenacycoctaphane-14,16,34,36,54,56,74,76-octol (PIN)
read (2r,4r,6r,8r)-2,4,6,8-tetranonyl-1,3,5,7(1,3)-tetrabenzenacycoctaphane-14,16,34,36,54,56,74,76-octol (PIN)
Page 1271, P-93.5.5.3, lines 3/4. [corrected 4 September 2024]
For ...present, all descriptors are must be cited (see P-91.2.2).
read ...present, all descriptors of double bonds in rings of more than seven nodes are cited (see P-91.2.2)
Page 1271, P-93.5.5.3, example 2. [corrected 4 September 2024]
For (2E)-1(2,6)-pyridina-7(1,3)-benzenacyclododecaphan-2-ene (PIN)
read (2E)-11,14,15,16-tetrahydro-1(2,6)-pyridina-7(1,3)-benzenacyclododecaphan-2-ene (PIN)
Page 1272, P-93.5.6.1, Fig. 9.2, third oblong. [corrected 2 September 2020]
For Is the resulting molecule chiral
read Is the resulting molecule chiral ?
Page 1272, P-93.5.6.1, Fig. 9.2, second ellipse. [corrected 2 September 2020]
For Noninherently chiral substitution pattern
read Type 3 Noninherently chiral substitution pattern
Page 1273, P-93.5.6.1, Fig. 9.2, third ellipse. [corrected 2 September 2020]
For Achiral substitution pattern; substitution units are only in substituents
read Type 4 Achiral substitution pattern; stereogenic units are only in substituents
Page 1273, P-93.5.6.2, line 3. [corrected 7 November 2018]
For ...(C76-D2)[5,6}fullerene shown below...
read ...(C76-D2)[5,6]fullerene shown below...
Page 1275, P-93.5.6.3, example. [corrected 3 October 2018]
For ...(C60-Ih)[5,6]-fullerene (PIN)
read ...(C60-Ih)[5,6]-fullerene (PIN)
Page 1277, P-93.5.6.5, example. [corrected 10 October 2018]
For ...[5,6]fullerene-3′,3′,3′′,3′′-tetracarboxylate
read ...[5,6]fullerene-3′,3′,3′′,3′′-tetracarboxylate (PIN)
Replace the structure with:
Page 1278, P-93.5.6.6, explanation line 4. (corrected 27 November 2019)
For tetrakis[(1R)-1-phenylbutyl](f,sA-3′H,3′′H-di...
read tetrakis[(1R)-1-phenylbutyl] (f,sA-3′H,3′′H-di... [add space in name]
Page 1279, P-93.5.7.1, example 1.
For (1M)-6,6′-dinitro-[1,1′-biphenyl]-2,2′-dicarboxylic acid (PIN)
read (1M)-6,6′-dinitro[1,1′-biphenyl]-2,2′-dicarboxylic acid (PIN)
for (1Ra)-6,6′-dinitro-[1,1′-biphenyl]-2,2′-dicarboxylic acid
read (1Ra)-6,6′-dinitro[1,1′-biphenyl]-2,2′-dicarboxylic acid
Page 1279, P-93.5.7.1, example 2.
For (1P)-2′,5′-dimethyl-6-nitro-[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1P)-2′,5′-dimethyl-6-nitro[1,1′-biphenyl]-2-carboxylic acid (PIN)
for (1Sa)-2′,5′-dimethyl-6-nitro-[1,1′-biphenyl]-2-carboxylic acid
read (1Sa)-2′,5′-dimethyl-6-nitro[1,1′-biphenyl]-2-carboxylic acid
Page 1279, P-93.5.7.1, example 3.
For (1M)-2′,6-diamino-6′-methoxy-[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1M)-2′,6-diamino-6′-methoxy[1,1′-biphenyl]-2-carboxylic acid (PIN)
for (1Ra)-2′,6-diamino-6′-methoxy-[1,1′-biphenyl]-2-carboxylic acid
read (1Ra)-2′,6-diamino-6′-methoxy[1,1′-biphenyl]-2-carboxylic acid
Page 1280, P-93.5.7.1, example 1 on this page.
For (2P)-2-[2′-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol (PIN)
read (2P)-2-[2-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol (PIN)
for (2Sa)-2-[2′-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol
read (2Sa)-2-[2-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol
replace structure with:
Page 1280, P-93.5.7.2, example on this page.
For [1S,1′s,2S,4S,4′R]-4,4′-dimethyl-1,1′-bi(cyclohexan)-2-ol (PIN)
read (1S,1′s,2S,4S,4′R)-4,4′-dimethyl[1,1′-bi(cyclohexan)]-2-ol (PIN)
Page 1281, P-93.5.7.2, example 1 on this page.
For (a) [1s,1′s,4s,4s′]-4,4′-dimethyl-1,1′-bi(cyclohexane) (PIN)
read (a) (1s,1′s,4s,4′s)-4,4′-dimethyl-1,1′-bi(cyclohexane) (PIN)
for (b) [1(4)cis,1′(4′)cis]-4,4′-dimethyl-1,1′-bi(cyclohexane)
read (b) [1(4)-cis,1′(4′)-cis]-4,4′-dimethyl-1,1′-bi(cyclohexane)
Page 1281, P-93.5.7.2, example 2 on this page.
For [11s,14s,21r,24r,31s,34s]-14,34-dimethyl-11,21:24,31-tercyclohexane (PIN)
read (11s,14s,21r,24r,31s,34s)-14,34-dimethyl-11,21:24,31-tercyclohexane (PIN)
for [11(14)cis,21(24)trans,31(34)cis]-14,34-dimethyl-11,21:24,31-tercyclohexane
read [11(14)-cis,21(24)-trans,31(34)-cis]-14,34-dimethyl-11,21:24,31-tercyclohexane
Page 1281, P-93.5.7.2, example 3 on this page.
For (1s,1′r,4s,4′r)-4-bromo-4′-butyl-4-(4-ethylphenyl)-1,1′-bi(cyclohexane) (PIN)
read (1s,1′s,4s,4′r)-4-bromo-4′-butyl-4-(4-ethylphenyl)-1,1′-bi(cyclohexane)
add (11s,14r,21s,24s)-24-bromo-14-butyl-34-ethyl-11,12,13,14,15,16,21,22,23,24,25,26-dodecahydro-11,21:24,31-terphenyl (PIN)
add (1s,1′s,4r,4′s)-4′-bromo-4-butyl-4′′-ethyl-1,1′,2,2′,3,3′,4,4′,5,5′,6,6′-dodecahydro-1,1′:4′,1′′-terphenyl
Page 1281, P-93.5.7.2, last example on this page. [modified 26 June 2019]
For (1E)-1-[(1′S,1r,4S)-[1,1′-bi(cyclohexan)-3′-en-4-yl]-N-[(1r,4r)-4-phenylcyclohexyl]methan-1-imine (PIN)
read (1E)-1-{(1r,1′S,4S)-[1,1′-bi(cyclohexan)]-3′-en-4-yl}-N-[(1r,4S)-4-phenylcyclohexyl]methanimine (PIN)
for (1E)-1-[(1′R,1(4)-trans)-1,1′-bi(cyclohexan)-3′-en-4-yl]-N-[(trans)-4-phenylcyclohexyl]methanimine
read (E)-1-{1(4)-trans-(1′S)-[1,1′-bi(cyclohexan)]-3′-en-4-yl}-N-(trans-4-phenylcyclohexyl)methanimine
replace structure with:
Page 1282, P-93.5.7.3, example 1.
For (3Z)-1,1′-bicyclooct-3-ene (PIN)
read (3Z)-[1,1′-bi(cyclooctan)]-3-ene (PIN)
Page 1282, P-93.5.7.3, example 2.
For (1Z,2′Z,3Z,5Z,7Z)-bicycloocta-1,2′,3,5,7-pentaene (PIN)
read (1Z,2′Z,3Z,5Z,7Z)-[1,1′-bi(cyclooctane)]-1,2′,3,5,7-pentaene (PIN)
Page 1282, P-93.6, example on this page. [corrected 12 September 2018]
For (2R)-1-[(1r,4R)-4-methylcyclohexyl]-3-[(1s,4S)-4-methylcyclohexyl]propan-2-ol (PIN)
read (2R)-1-[(1r,4S)-4-methylcyclohexyl]-3-[(1s,4S)-4-methylcyclohexyl]propan-2-ol (PIN)
add (2R)-1-(cis-4-methylcyclohexyl)-3-(trans-4-methylcyclohexyl)propan-2-ol
replace structure with:
Page 1283, P-93.6, first digraph. [corrected 12 September 2018]
Replace structure with:
for simplified digraph to for the configuration ‘R’ at C-1.
read simplified digraph for the configuration ‘S’ at C-1 (sequence rule 4b like > unlike).
Page 1284, P-93.6, example 2.
For (11R,12S,14S,4E,7E,111S,112R,114R)-12,112-bis{[(1E)-2-phenyleth-1-en-1-yl]oxy}-2,10-dioxa-3,9(1,4)-dibenzena-1,11(1,4)-dicyclohexanaundecaphane-4,7-diene-14,114-dicarboxylic acid (PIN: a phane name)
read (2E,51S,52R,55S,8E,11E,151S,152R,154R,17E)-4,6,10,14,16-pentaoxa-1,19(1),7,13(1,4)-tetrabenzena-5,15(1,2)-dicyclohexananonadecaphane-2,8,11,17-tetraene-55,154-dicarboxylic acid (PIN: a phane name)
replace structure with:
Page 1284, P-93.6, example 3.
For (11S,12R,14R,4Z,7E,111R,112S,114S)-12,112-bis{[(1E)-2-phenyleth-1-en-1-yl]oxy}-2,10-dioxa-3,9(1,4)-dibenzena-1,11(1,4)-dicyclohexanaundecaphane-4,7-diene-14,114-dicarboxylic acid (PIN: a phane name)
read (2E,51R,52S,55R,8Z,11E,151R,152S,154S,17E)-4,6,10,14,16-pentaoxa-1,19(1),7,13(1,4)-tetrabenzena-5,15(1,2)-dicyclohexananonadecaphane-2,8,11,17-tetraene-55,154-dicarboxylic acid (PIN: a phane name)
replace structure with:
Page 1285, P-93.6, example 4.
For (2S,2′S)-2,2′-oxybis[(1E)-ethene-2,1-diyl-4,1-phenylene]dipropanoic acid (PIN)
read (2S,2′S)-2,2′-{oxybis[(1E)-ethene-2,1-diyl-4,1-phenylene]}dipropanoic acid (PIN)
Page 1285, P-93.6, example 5. [modified 24 October 2018]
For (2R)-2-bromo-2-(4-{(1E)-2-[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}eth-1-en-1-yloxy]eth-1-en-1-yl}phenyl]propanoic acid
read (2R)-2-bromo-2-{4-[(1E)-2-{[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethen-1-yl]oxy}ethen-1-yl]phenyl}propanoic acid
replace the structure with:
Page 1285, P-93.6, Example 6. [modified 30 September 2020]
For (2S)-2-(4-{(1Z)-2-[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethenyloxy]ethenyl}phenyl)propanoic acid (PIN;
read (2S)-2-{4-[(E)-2-{(Z)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethen-1-yl]oxy}ethen-1-yl]phenyl}propanoic acid (PIN;
Page 1287, P-94.2.1, top diagram.
Replace diagram with:
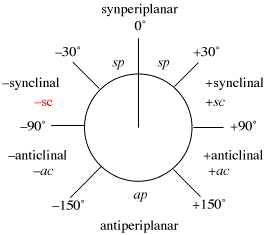
Page 1291-2, P-94.3.2.6, Example 1. [corrected 30 September 2020]
Replace the structures with:
Page 1292, P-94.3.2.6, Example 2. [corrected 30 September 2020]
Replace the structures with:
Page 1298, P-101.2.5, example 1. [corrected 12 August 2020]
Replace the structure with:
Page 1298, P-101.2.6, lines 3-5. [corrected 21 October 2020
For ...further specification, for example the ‘5α’ position in steroids is usually not defined but it must, however, be defined in preferred IUPAC names. When a planar...
read ...further specification. All chirality must be defined so that for example with a steroid the stereochemistry at ‘C-5’, when relevant, is indicated by α, β or ξ. When a planar...
Page 1300, P-101.2.6.1.2, example. [corrected 17 October 2018]
Replace the structures with:
Page 1301, Table 10.1 (a).
For orynan read corynan
for formosana read formosanan
for lythrancine read lythran
for oxyacathan read oxyacanthan
for solanidine read solanidane
for strychinidine read strychnidine
for vincaleuckoblastine read vincaleukoblastine
add curan, lythranidine, nupharidine, and vincane
Page 1301, Table 10.1 (c).
For pimerane read pimarane
for himalachane read himachalane
add germacrane
Page 1301, Table 10.1 (c).
For prostantane read protostane
for ritinal read retinal
Page 1302, Table 10.1, footnote.
For Φ (phi) read ![]() (phi)
(phi)
for Ψ (psi) read ψ (psi)
Page 1304, P-101.3.1.1, example 1 on this page.
Replace structure 1 with:
Page 1304, P-101.3.1.1, example 3 on this page. [corrected 26 September 2018]
Replace the structure with:
Page 1304, P-101.3.1.1, structure 4 on this page.
For (1R,4s,7S)-4-ethyl-4,7-dimethylcyclodecane
read (1R,4s,7S)-4-ethyl-1,7-dimethylcyclodecane (position 1 is position 10 for germacrane)
Page 1306, P-101.3.2.2.1, example 2.
For 19a-homo-5β-pregnane (PIN)
read 19a-homo-5β-pregnane
Page 1306, P-101.3.2.2.1, example 3.
For 16a-homo-5α-pregnane (PIN)
read 16a-homo-5α-pregnane
Page 1306, P-101.3.2.2.1, last example.
Replace structure with:
Page 1307, P-101.3.2.2.2, last example.
Replace structure with:
Page 1309, P-101.3.3, structure 1. [corrected 17 October 2018]
Replace the structure with:
Page 1312, P101.3.5.1, example 2. [corrected 14 November 2018]
For (3αH)-5(4→3)-abeo-podocarpane
read (3αH)-5(4→3)-abeopodocarpane
Page 1313, P-101.3.5.2, example 1. [corrected 23 September 2020]
Replace the structure with:
Page 1313, P-101.3.5.2, example 2. [corrected 23 September 2020]
Replace the structure with:
Page 1315, P-101.3.7.2, line 11. [corrected 23 September 2020]
For ...prefixes ‘cyclo’, ‘seco’, ‘homo’, and ‘nor’. In general...
read ...prefixes ‘cyclo’, ‘seco’, ‘apo’, ‘homo’, and ‘nor’. In general...
Page 1316, P-101.3.7.2, example 4. [corrected 17 April 2019]
For 3α,5-cyclo-9,10-seco-5α-androstane
read 3α,5α-cyclo-9,10-seco-5α-androstane
Page 1318, P-101.4.2, example 1.
Replace the structure with:
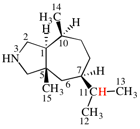
Page 1321, P-101.5.1.1, example 3. [corrected 9 January 2019]
Replace the structure with:
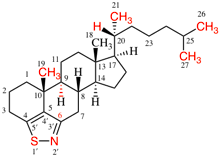
Page 1325, P-101.5.2, example 1 on this page.
Replace structure with:
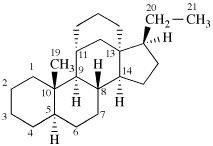
Page 1325, P-101.5.2, example 3 on this page. [corrected 17 April 2019]
For (7α,8α,8′β,9′α)-7,9a′:8′,9-diepoxy-7-oxa-9a′-homo-8,9′-neolignane
read (7α,8α,8′β,9′α)-7,9a′:8′,9-diepoxy-7′-oxa-9a′-homo-8,9′-neolignane
Page 1328, P-101.6.2, example 2. [corrected 17 October 2018]
Replace the structure with:
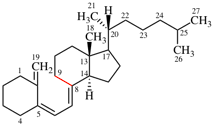
Page 1329, P-101.6.2, example 3 on this page. [corrected 17 April 2019]
For 1-[(1E,3S)-2,3-dimethyl-4-phenylbut-1-en-1-yl]benzene
read [(1E,3S)-2,3-dimethyl-4-phenylbut-1-en-1-yl]benzene
for 1,1′-[(1E,3S)-2,3-dimethylbutane-1,4-diyl]dibenzene
read 1,1′-[(1E,3S)-2,3-dimethylbut-1-ene-1,4-diyl]dibenzene
Page 1329, P-101.6.3, example. [corrected 7 November 2018]
For (all-trans)-retinal
read all-trans-retinal
Page 1330, P-101.6.4, second example.
Both structures are wrong, they should be:
Page 1331, P-101.6.5, example 2. [corrected 17 October 2018]
Replace the structure with:
Page 1333, P-101.7.1.1.1, example. (modified 27 November 2019)
For 8α-ethyl-5α-eudesmane
read 8α-ethyleudesmane
Page 1334, P-101.7.1.1.2, example. [corrected 23 September 2020]
Replace the structure with:
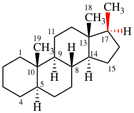
Page 1334, P-101.7.1.1.3, example 1. [corrected 24 October 2018]
Replace the structure with:
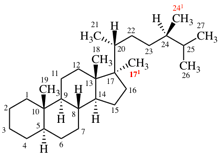
Page 1335, P-101.7.1.1.4, example.
For [not (21-nor-5α-pregnan-17α-yl))methanol]
read [not (21-nor-5α-pregnan-17α-yl)methanol]
Page 1335, P-101.7.1.2, example 2. [corrected 17 October 2018]
Replace the structure with:
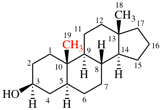
Page 1336, P-101.7.1.2, example 4 on this page. [corrected 23 September 2020]
For 7β-amino-3-methyl-2,3-didehydrocepham-2-carboxylic acid
read 7β-amino-3-methyl-3,4-didehydrocepham-4-carboxylic acid
replace the structure with:
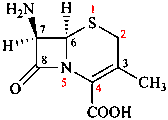
Page 1339, P-101.7.4, example 1. [corrected 24 October 2018]
Replace the structure with:
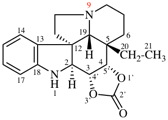
Page 1339, P-101.7.4, examples 2. [modified 24 October 2018]
For 19,21-epoxyspermidin-21-one
read 19,21-epoxyaspidospermidin-21-one
replace the structure with:
Page 1339, P-101.7.4, example 3. [modified 26 June 2019]
For (3αH,4αH)-2′,2′-dimethyl-3,4-dihydro-[1,3]-dioxolo[4′,5′:3,4]matridine
read (3αH,4αH)-2′,2′-dimethyl-3,4-dihydro[1,3]dioxolo[4′,5′:3,4]matridine
For propane-2-one matridine-3β,4β-diyl acetal
read propane-2-one matridine-3β,4β-diyl ketal
for acetone matridine-3β,4β-diyl acetal
read acetone matridine-3β,4β-diyl ketal
Page 1339, P-101.7.4, example 3. [corrected 23 September 2020]
Replace the structure with:
Page 1341, P-101.7.5, scheme at top of this page.
Replace the structures with:
Page 1343, P-101.8.4, example 3 (structure II). [corrected 26 September 2018]
Replace the structure with:
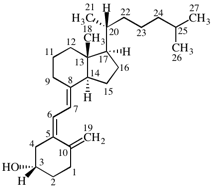 [no hydrogen at C-8]
[no hydrogen at C-8]
Page 1346, P-102.2.1, table 10.2.
For Retained and systematic carbohydrate names (with recommended three-letter abbreviations in parantheses) and structures...
read Retained and systematic carbohydrate names and structures...
Page 1346, P-102.2.1, table 10.2 structure 1, D-glyceraldehyde.
For 2,3-dihydroxypropanal
read (2R)-2,3-dihydroxypropanal
replace the structure with:
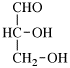
Page 1347, P-102.2.1, table 10.2 structure 2 on this page, D-arabinose.
Replace the structure with:
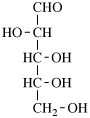
Page 1347, P-102.2.1, table 10.2 structure 5 on this page, D-allose.
Replace the structure with:
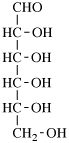
Page 1347, P-102.2.1, table 10.2 structure 6 on this page, D-altrose.
For D-alto-hexose
read D-altro-hexose
replace the structure with:
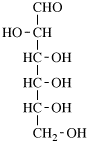
Page 1347, P-102.2.1, table 10.2 structure 7 on this page, D-glucose.
Replace the structure with:
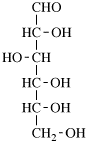
Page 1347, P-102.2.1, table 10.2 structure 8 on this page, D-mannose.
Replace the structure with:
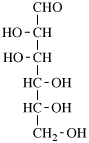
Page 1347, P-102.2.1, table 10.2 structure 9 on this page, D-gulose.
Replace the structure with:
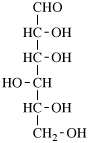
Page 1347, P-102.2.1, table 10.2 structure 10 on this page, D-idose.
Replace the structure with:
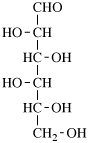
Page 1347, P-102.2.1, table 10.2 structure 11 on this page, D-galactose.
Replace the structure with:
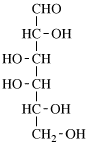
Page 1347, P-102.2.1, table 10.2 structure 12 on this page, D-talose.
Replace the structure with:
Page 1348, P-102.2.1, Table 10.3 structure 2, D-erythrulose.
For D-ethryulose
read D-erythrulose
replace the structure with:
Page 1348, P-102.2.1, Table 10.3 structure 3, D-ribulose.
Replace the structure with:
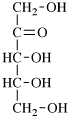
Page 1348, P-102.2.1, Table 10.3 structure 4, D-xylulose.
Replace the structure with:
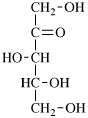
Page 1348, P-102.2.1, Table 10.3 structure 6, D-fructose.
Replace the structure with:
Page 1349, P-102.3.1, line 5.
For Various representations ‘b’, ‘c’, ‘d’, ‘e’ and ‘f’
read Various representations ‘b’, ‘c’, ‘d’ and ‘e’
Page 1349, P-102.3.1, structure a.
Replace structure with:
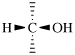
Page 1350, P-102.3.3, example 4.
Replace structure with:
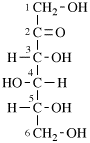
Page 1352, P-102.3.4.1.2, lines 3/4. [corrected 23 September 2020]
For ...the second one: step (c) is the reorientation of carbon C-5...
read ...the second reorientation, step (c), is the rotation at carbon C-5...
Page 1353, P-102.3.4.2.2, example 3, left structure. [corrected 18 May 2023]
replace the structure with:
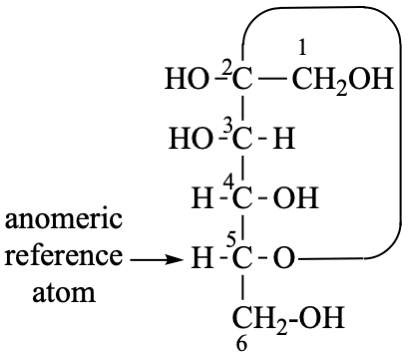
Page 1358, P-102.5.1.1.4, right hand structure.
Replace structure with:
Page 1358, P-102.5.2.2, line 1 and 2.
For ...Table 10.3, configuration being designated by the appropriate CIP stereodescriptors ‘R’, ‘S’, ‘r’, etc.
read ...Table 10.3; configuration is specified by a ‘D’ or ‘L’ stereodescriptor, as defined in P-102.3.2.
Page 1359, P-102.5.2.3, example 2.
Delete this example as it is a duplicate of example 3.
Page 1361, P-102.5.2.3, example 1 left structure on this page. [corrected 3 October 2018]
For [not D-threo-D-allo-non-5-ulose; ...
read [not D-threo-D-allo-non-5-ulose; ...
Page 1362, P-102.5.3.4, example 1.
For (1ξ,4S,5R)-5-(hydroxymethyl)oxolane-2,4-diol
read (2ξ,4S,5R)-5-(hydroxymethyl)oxolane-2,4-diol
Page 1371, P-102.5.6.3.2, example 3.
For (2S,3R,4S,5S,6R)-3-bromo-3-chloro-6-(hydroxymethyl)oxane-2,3,4-triol
read (2S,3R,4S,5S,6R)-3-bromo-3-chloro-6-(hydroxymethyl)oxane-2,4,5-triol
Page 1371, P-102.5.6.3.2, example 4.
For (2S,3S,4S,5R,6R)-3-acetamido-6-[(acetyloxy)methyl]-2-fluorooxane-2,4,5-triyl triacetate
read (2S,3S,4S,5R,6R)-3-acetamido-6-[(acetyloxy)methyl]-2-fluorooxane-3,4,5-triyl triacetate
Page 1373, P-102.5.6.5.2, line 1. [corrected 18 May 2023]
For The prefix ‘meso’ must be included in the preferred name of erythritol....
read The prefix ‘meso’ may be included in the name of erythritol....
Page 1374, P-102.5.6.5.2, example 2.
For
(2R,3S,4r,5R,6S)-heptane-1,2,3,4,5,6,7-aldito
read (2R,3S,4r,5R,6S)-heptane-1,2,3,4,5,6,7-heptol
Page 1380, P-102.5.6.6.4.3, example 3.
For (2R,3S,4R,5R,6R)-3,4,5,6-tetra(acetyloxy)-2-bromooxane-2-carboxylic acid
read (2R,3S,4R,5R,6R)-3,4,5,6-tetrakis(acetyloxy)-2-bromooxane-2-carboxylic acid
Page 1381, P-102.5.6.6.5.1, line 3/4. [corrected 18 May 2023]
For ...The descriptor ‘meso’ must be added for sake of clarity...
read ...The descriptor ‘meso’ may be added for the sake of clarity...
Page 1382, P-102.5.6.6.5.3, example 3.
For D-glucar-1-amic acid
read D-glucar-6-amic acid
replace the structure with:
Page 1382, P-102.5.6.7.1, example 1.
For (2R,3R,4R,5S)-2-(hydroxymethyl)oxane-3,4,5-triol
read (2R,3R,4R,5S)-2-(hydroxymethyl)oxane-3,4,5-triol
Page 1383, P-102.6, line 2.
For P-102.6.2 Monosaccharides as substituent groups
read P-102.6.2 Substituent groups other than glycosyl groups
Page 1384, P-102.6.1, line 2 on this page.
Delete P-102.6.1.6 Substituent groups other than glycosyl groups
Page 1385, P-102.6.1.2, example 2. [corrected 23 September 2020]
Replace the structure with:
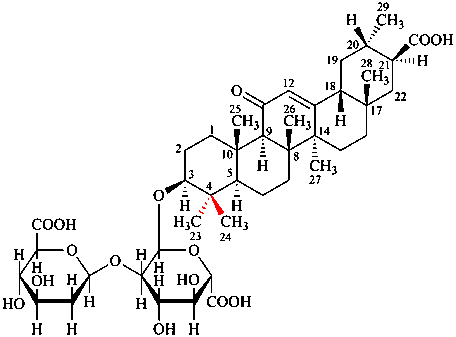
Page 1386, P-102.6.1.2, example on this page.
For 4-[(R)-2-amino-3-hydroxy-2-methylpropanamido]-N-{1-[(2R,5S,6R)-(5-{[4,6-dideoxy-4-(dimethylamino)-α-D-glucopyranosyl]oxy}-6-methyloxan-2-yl)]-2-oxo-1,2-dihydropyrimidin-4-yl}benzamide
read
4-[(2R)-2-amino-3-hydroxy-2-methylpropanamido]-N-{1-[(2R,5S,6R)-5-{[4,6-dideoxy-4-(dimethylamino)-α-D-glucopyranosyl]oxy}-6-methyloxan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-yl}benzamide
Page 1387, P-102.6.1.5, example 1. [corrected 3 October 2018]
For 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
read 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
Page 1387, P-102.6.1.6, section number.
For P-102.6.1.6
read P-102.6.2
Page 1388, P-102.6.1.6, last example.
For 2-(β-D-glucopyranos-2-O-yl)acetic acid
read (β-D-glucopyranos-2-O-yl)acetic acid
Page 1389, P-102.7.1.1, example note line 2.
For ... gluco precedes fructo in the alphabetical...
read ... fructo precedes gluco in the alphabetical...
Page 1392, P-103.1.1.1, table 10.4 entry 2. [corrected 27 July 2018]
Delete line of space after arginine
for 2-amino-4-carbamimidamidopentanoic acid
read 2-amino-5-(carbamimidoylamino)pentanoic acid
Page 1392, P-103.1.1.1, Table 10.4, entry 4 aspartic acid [corrected 28 April 2023]
For 2-aminobutanedioic acid
read aminobutanedioic acid
Page 1392, P-103.1.1.1 Table 10.4, entry 6 (glutamine).
Replace the structure with:
H2N-CO-[CH2]2-CH(NH2)-COOH
Page 1392, P-103.1.1.1, table 10.4 entry 8. (glycine)
For 2-aminoacetic acid
read aminoacetic acid
Page 1394, P-103.1.1.2, table 10.5, entry 2, symbol.
For alle
read aIle
Page 1394, P-103.1.1.2, Table 10.5, entry 4.
For 2-amino-5-oxopentanoic acid
read 2-amino-6-oxohexanoic acid
Page 1394, P-103.11.3, Table 10.5 entry 6.
For S-(2-amino-2-carboxyethyl)homosysteine
read S-(2-amino-2-carboxyethyl)homocysteine
Page 1395, P-103.1.1.2, table 10.5, example 5 on this page.
Replace structure with:
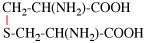
Page 1395, P-103.1.1.2, table 10.5, last example on this page.
For O-(4-hydroxy-3,5-diiodophenyl)-3,5-iodotyrosine
read O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodotyrosine
Page 1396, P-103.1.1.3, line 7. [corrected 17 April 2019]
For ...Ahx. The CIP stereodescriptors R and S are used...
read ...Ahx. The stereodescriptors D and L are used...
Page 1400, P-103.2.1, example 5. [corrected 17 October 2018]
Replace the structure with:
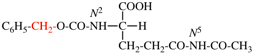
Page 1400, P-103.2.1, example 6. [corrected 24 October 2018]
Replace the structure with:
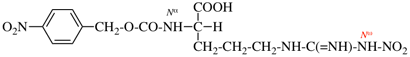
Page 1401, P-103.2.2.2, last example on this page. [corrected 17 April 2019]
For (4-amino-5-carboxypentyl)amino
read (5-amino-5-carboxypentyl)amino
Page 1405, P-103.2.4.3.2, line 1. [corrected 26 June 2019]
For The singly charged cations of asparagine, glutamine, and lysine...
read The singly charged cations of arginine, histidine, and lysine...
Page 1406, P-103.2.7, line 1. [corrected 19 September 2018]
For ...and other nitrogeneous analogues derivatives...
read ...and other nitrogenous analogues derivatives...
Page 1407, P-103.2.7, title.
For P-103.2.7 Alcohols, aldehyde, ketones, and nitriles
read P-103.2.8 Alcohols, aldehyde, ketones, and nitriles
Page 1407, P-103.2.7, example 1.
For aminoacetamide
read 2-aminoacetamide
Page 1407, P-103.2.7, example 2.
For aspartic 1-amide
read asparaginamide
Page 1407, P-103.2.7, example 3.
For glutamic 1-amide
read glutaminamide
Page 1407, P-103.2.7, example 4 on this page. [corrected 23 September 2020]
For 2-amino-1-N-phenylacetamide
read 2-amino-N1-phenylacetamide
Page 1408, P-103.3.1, structure.
Replace the structure with:
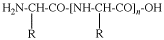
Page 1409, P-103.3.4, line 9.
For L-leucine by L-alanine
read L-leucine by DL-alanine
Page 1409, P.103.3.5, line 4. [corrected 17 April 2019]
For ...The retained names angiotensine II, bradikinin...
read ...The retained names angiotensin II, bradikinin...
Page 1410, P-103.3.5, example 2 on this page.
Replace structure with:

Page 1410, P-103.3.5.1, example 2. [corrected 17 April 2019]
For Ile5,Ala5]angiotensin II
read [Ile5,Ala7]angiotensin II
Page 1411, P-103.3.5.1.1, example 1.
Replace structure with:

Page 1411, P-103.3.5.1.1, example 2.
Replace structure with:

Page 1411, P-103.3.5.1.2, example.
Replace structure with:

Page 1412, P-103.3.5.2.1, example 3. [modified 26 June 2019]
For [A21-glycine]insulinyl-B-arginine,arginine]insulin (human)
read [A21-glycine]insulin-B30-yl-L-arginyl-L-arginine (human)
replace structure with:

move example to P-103.3.5.2.2
Page 1412, P-103.3.5.2.2, example 1.
For radykininylleucine
read bradykininylleucine
for bradykinin-Leu
read bradykininyl-Leu
Page 1412, P-103.3.5.2.2, example 2.
For insulintl-B-arginine.ardinine (human)
read insulin-B30-yl-L-arginyl-L-arginine (human)
Replace structure with:

Page 1414, P-103.3.5.4, example 1 on this page.
For des-B1-phynylalanine-insulin (cattle)
read des-B1-phenylalanine-insulin (cattle)
for des-PheB1-insulin(cattle)
read des-PheB1-insulin (cattle)
Replace structure with:

Page 1416, P-104.2.2, example 1. [modified 2 January 2019]
For (1R,2R,3R,5S)-cyclohexane-1,2,3,5-tetrol
read (1R,2R,3R,5R)-cyclohexane-1,2,3,5-tetrol
replace the structure with:
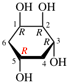
Page 1417, P-104.2.3, example 1.
For (1R,2R,3R,5S)-cyclohexane-1,2,3,5-tetrol
read (1R,2R,3R,5R)-cyclohexane-1,2,3,5-tetrol
Page 1419, P-104.3.1, example 3 on this page. [modified 12 August 2020]
For 2-methyl-myo-inositol
read 2-C-methyl-myo-inositol
for (1s,2S,3R,4r,5S,6R)-1-methylcyclohexane-1,2,3,4,5,6-hexol
read (1s,2R,3S,4s,5R,6S)-1-methylcyclohexane-1,2,3,4,5,6-hexol
Page 1419, P-104.3.1, example 4 on this page. [modified 21 October 2020
Delete left hand structure.
delete I and II
add (not 2-carboxy-2-deoxy-myo-inositol; COOH is senior to OH)
Page 1422, P-105.2.1, example 2.
For 4-amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(methoxymethyl)oxolan-2-yl-5-iodopyrimidin-2(1H)-one
read 4-amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(methoxymethyl)oxolan-2-yl]-5-iodopyrimidin-2(1H)-one
Page 1423, P-105.2.1, example 3 on this page. [corrected 21 October 2020
For N-(2-hydroxyethyl)-5′-S-methyl-5′-thioguanosine
read N2-(2-hydroxyethyl)-5′-S-methyl-5′-thioguanosine
Page 1424, P-105.2.1, example on this page. [corrected 19 September 2018]
For 2′,3′,5′-tri-O-acetylguanosine
read 2′,3′,5′-tri-O-acetyladenosine
for guanosine 2′,3′,5′-triacetate
read adenosine 2′,3′,5′-triacetate
Page 1428, P-106.3.1, example 1 on this page.
For N6-(N-propylcarbamoyl)-5′-adenylic acid
read N6-(propylcarbamoyl)-5′-adenylic acid
Page 1430, P-106.3.3, example on this page.
Replace the structure with:
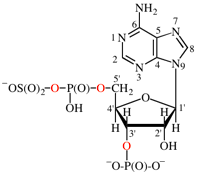
Page 1432, P-107.2, example 2. [corrected 28 October 2016]
For (2S)-2-O-acetyl-1-O-hexadecanoyl-3-O-(9Z)-octadec-9-enoylglycerol
read (2S)-2-O-acetyl-1-O-hexadecanoyl-3-O-[(9Z)-octadec-9-enoyl]glycerol
Page 1433, P-107.3.1, structure 1.
For (for a discussion and examples of ‘sn’ see P-107.3.1.2)
read (for a discussion and examples of ‘sn’ see P-107.3.2)
Page 1433, P-107.3.2, line 9. [corrected 17 April 2019]
For ...and the stereodescriptor ‘X’ may be used...
read ...and the stereodescriptor ‘Ξ’ may be used...
Page 1434, P-107.3.3, example. [modified 22 April 2020]
For 2,3-bis(octadecanoyl)-sn-glycero-3-phospho-L-serine
read 1,2-di(octadecanoyl)-sn-glycero-3-phospho-L-serine
for O-({[(2R)-2,3-bis(octadecanoyloxy)propoxy]hydroxyphosphoryl})-L-serine
read
O-{[(2R)-2,3-bis(octadecanoyloxy)propoxy]hydroxyphosphoryl}-L-serine
replace the structure with:
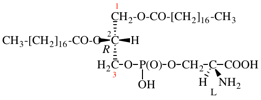
Page 1435, P-107.3.5, example.
For 2,3-bis(hexadecanoyl)-sn-glycero-3-phosphoethanolamine
read 1,2-di(hexadecanoyl)-sn-glycero-3-phosphoethanolamine.
Page 1435, P-107.3.5 example [corrected 28 April 2023]
For (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl dihexadecanoate
read (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl di(hexadecanoate)
Page 1435, P-107.3.6 example. [modified 28 April 2023]
For L-myo-inositol 2-[(2R)-2,3-bis(hexadecanoyloxy)propyl hydrogen phosphate
read L-myo-inositol 2-[(2R)-2,3-bis(hexadecanoyloxy)propyl hydrogen phosphate]
for (2R)-3-[({[(1s,2R,3S,4s,5R,6S)-hydroxy-2,3,4,5,6-pentahydroxycyclohexyl]oxy}hydroxyphosphoryl)oxy]propane-1,2-diyl dihexadecanoate
read (2R)-3-[(hydroxy{[(1s,2R,3R,4s,5S,6S)-2,3,4,5,6-pentahydroxycyclohexyl]oxy}phosphoryl)oxy]propane-1,2-diyl di(hexadecanoate)
Page 1436, P-107.4.2 example. [modified 28 April 2023]
For (2S)-3-(O-β-D-galactopyranosyl)propane-1,2-diyl dioctadecanoate
read (2S)-3-(β-D-galactopyranosyloxy)propane-1,2-diyl di(octadecanoate)
replace the structure with:
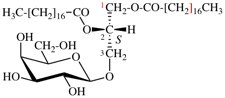
Page 1443, appendix 1, column 2, line 14.
For Ru ruthenica
read Ru ruthena
Page 1444, appendix 1, column 1, line 17.
For ‘Yt’
read ‘Yb’
Page 1444, Appendix 1, column 2, elements 15 and 16. [corrected 23 September 2020]
Reverse the order of Na and K
Page 1447, appendix 2, line 1
For aminocarbonimidoyl* = carbamimidoyl
read C-aminocarbonimidoyl = carbamimidoyl*
Page 1447, appendix 2, line 16
For = aminocarbonimidoyl
read = C-aminocarbonimidoyl
Page 1448, appendix 2, line 20. [corrected 14 November 2018]
For = 1 anthryl
read 1-anthryl
Page 1449, appendix 2, line 18.
For arsono* = dihydroxynitroryl
read arsono* = dihydroxyarsoryl
Page 1450, appendix 2, line 6. [corrected 24 April 2019]
For azinoyl* = dihydroazoryl
read azinoyl* = dihydronitroryl
Page 1450, appendix 2, line 15. [modified 20 January 2021]
For azoxy* –N(O)=N– or –N=N(O)– P-68.3.1.3.3.1
read NON-azoxy –N(O)=N– or –N=N(O)– P-68.3.1.3.3.1
add ONN-azoxy –N(O)=N– P-68.3.1.3.3.1
add NNO-azoxy –N=N(O)– P-68.3.1.3.3.1
Page 1450, appendix 2, lines 20-21. [modified 25 November 2020]
For benzenecarbonyl = benzoyl* = oxo(phenyl)methyl
read benzenecarbonyl = benzoyl* = oxo(phenyl)methyl = phenylcarbonyl
Page 1450, appendix 2, line 27. [corrected 24 April 2019]
For = benzenecarbothioylamino
read = (benzenecarbothioyl)amino
Page 1450, appendix 2, lines 22/23. [corrected 24 April 2019]
For benzenecarbothioamido* = benzenecarbothioylamino = thiobenzamido
read benzenecarbothioamido* = (benzenecarbothioyl)amino = thiobenzamido
Page 1451, appendix 2, lines 1/2. [corrected 24 April 2019]
For benzenecarboximidohydrazido* = 2-benzenecarboximidoylhydrazin-1-yl
read benzenecarboximidohydrazido* = 2-(benzenecarboximidoyl)hydrazin-1-yl
Page 1451, appendix 2, lines 5/6. [corrected 24 April 2019]
For 2-benzenecarboximidoylhydrazin-1-yl = benzenecarboximidohydrazido*
read 2-(benzenecarboximidoyl)hydrazin-1-yl = benzenecarboximidohydrazido*
Page 1451, appendix 2, lines 16-19. [corrected 24 April 2019]
For benzene-1,2-dicarbothioyl* = 1,2-phenylenebis(sulfanylidenemethylene) = phenylenebis(sulfanylidenemethylene) (not dithiophthaloyl)
read benzene-1,2-dicarbothioyl* = 1,2-phenylenebis(sulfanylidenemethylene) = 1,2-phenylenebis(thioxomethylene) (not dithiophthaloyl)
Page 1452, appendix 2, line 5. [corrected 24 April 2019]
For benzenesulfinohydrazonamido* = benzenesulfinohydrazonoylamino
read benzenesulfinohydrazonamido* = (benzenesulfinohydrazonoyl)amino
Page 1452, appendix 2, line 6. [corrected 24 April 2019]
For benzenesulfinohydrazonoylamino = benzenesulfinohydrazonamido*
read (benzenesulfinohydrazonoyl)amino = benzenesulfinohydrazonamido*
Page 1452, appendix 2, line 16, and move to under line 21. [corrected 24 February 2021]
For benzhydroximoyl: see N-hydroxybenzenecarboximidoyl*
read benzohydroximoyl = N-hydroxybenzenecarboximidoyl* = N-hydroxybenzimidoyl = benzenecarbohydroximoyl C6H5-C(=N-OH)– P-65.1.7.2.2
Page 1452, appendix 2, line 18. [corrected 24 April 2019]
For benzidino = [(4′-amino[1,1′-biphenyl]-4-yl)amino]*
read benzidino = (4′-amino[1,1′-biphenyl]-4-yl)amino*
Page 1452, appendix 2, lines 22/23. [modified 25 November 2020]
For benzoyl* = benzenecarbonyl = oxo(phenyl)methyl
read benzoyl* = benzenecarbonyl = oxo(phenyl)methyl = phenylcarbonyl
Page 1453, appendix 2, line 4. [corrected 24 April 2019]
For benzylidene* = phenylmethylidene
read benzylidene* = phenylmethylidene (not benzal)
Page 1453, appendix 2, column 2, line 16. [corrected 26 September 2018]
Replace structures with:

Page 1455, appendix 2, line 10. [corrected 24 April 2019]
For butan-1-ylidyne = butylidyne*
read butanylidyne = butylidyne*
Page 1455, appendix 2, lines 11/12. [modified 20 January 2021]
For butan-2-yloxy* = 1-methylpropoxy (not sec-butoxy)
read (butan-2-yl)oxy* = 1-methylpropoxy (not sec-butoxy; not sec-butyloxy)
Page 1455, appendix 2, column 2, structure 5 from bottom. [corrected 9 January 2019]
Replace structures with:
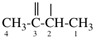
Page 1456, appendix 2, line 10/11. [modified 20 January 2021]
For tert-butoxy (unsubstituted*) = 2-methylpropan-2-yloxy* = 1,1-dimethylethoxy
read tert-butoxy* (unsubstituted) = (2-methylpropan-2-yl)oxy = 1,1-dimethylethoxy
Page 1456, appendix 2, line 19. [corrected 24 April 2019]
For butylidyne* = butan-1-ylidyne
read butylidyne* = butanylidyne
Page 1457, appendix 2, line 11.
For carbamimidoyl* = aminocarbonimidoyl
read carbamimidoyl* = C-aminocarbonimidoyl
Page 1457, appendix 2, line 17. [corrected 31 October 2018]
For carbamohydrazonoyl* = hydrazinylidene(amino)methyl
read carbamohydrazonoyl* = amino(hydrazinylidene)methyl
Page 1458, appendix 2, line 21, move to after line 24. [corrected 24 April 2019]
For carbononitridoperoxy = cyanoperoxy*
read carbononitridoylperoxy = cyanoperoxy*
Page 1459, appendix 2, line 23. [move after line 24]
For (carboxycarbonothioyl)sulfanyl)
read (carboxycarbonothioyl)sulfanyl
Page 1459, appendix 2, column 2, line 13.
For HOCO-CO-S–
read HOOC-CO-S–
Page 1460, appendix 2, line 1. [corrected 25 November 2020]
For chloro(amido)phosphoryl = phosphoroamidochloridoyl* (H2N)ClP(O)– P-67.1.4.1.1.4
read chloroamidophosphoryl: see phosphoramidochloridoyl*
Page 1460, appendix 2, line 20. [corrected 1 May 2019]
For cyano* = carbononitrodoyl
read cyano* = carbononitridoyl
Page 1460, appendix 2, line 21. [corrected 1 May 2019]
For cyanocarbonyl = carbocyanatidoyl*
read cyanocarbonyl = carbocyanidoyl*
Page 1460, appendix 2, lines 24-26. Correction deleted [11 November 2020]
Page 1460, appendix 2, lines 27-29. Correction deleted [11 November 2020]
Page 1460, appendix 2, line 30. [corrected 1 May 2019]
For cyanoperoxy* = carbononitridoperoxy
read cyanoperoxy* = carbononitridoylperoxy
Page 1462, appendix 2, line 1. [corrected 1 May 2019]
For decanedioyl* = 1,10-dioxodecanyl
read decanedioyl* = 1,10-dioxodecane-1,10-diyl
Page 1462, appendix 2, line 15.
For (NH2)P–
read (NH2)2P–
Page 1462, appendix 2, lines 26/27. [corrected 1 May 2019]
For diazenecarbohydrazido* = 2-diazenecarbonylhydrazin-1-yl (not carbazono)
read diazenecarbohydrazido* = 2-(diazenecarbonyl)hydrazin-1-yl (not carbazono)
Page 1462, appendix 2, line 28. [corrected 1 May 2019]
For diazenecarbonyldiazenyl*
read (diazenecarbonyl)diazenyl*
Page 1462, appendix 2, lines 29/30. [corrected 1 May 2019]
For 2-diazenecarbonylhydrazin-1-yl = diazenecarbohydrazido* (not carbazono)
read 2-(diazenecarbonyl)hydrazin-1-yl = diazenecarbohydrazido* (not carbazono)
Page 1462, Appendix 2, column 2, line 20. (corrected 27 November 2019)
For HN=NN-CO-N=NH–
read HN=NN-CO-N=N–
Page 1463, appendix 2, line 28. [corrected 1 May 2019]
For dihydroxycarbonimidoyl*
read C,N-dihydroxycarbonimidoyl*
Page 1463, appendix 2, column 2, structure 2. [corrected 9 January 2019]
For H2N=N–
read HN=N–
Page 1464, appendix 2, line 21. [modified 3 March 2021]
For dimethylammoniumylidene: see dimethylazaniumylidene*
read dimethylammoniumylidene: see N-methylmethanaminiumylidene*
Page 1464, appendix 2, line 25 and move to page 1485 after line 2. [modified 3 March 2021]
For dimethylazaniumylidene* (not dimethylammoniumylidene)
read N-methylmethanamiumylidene* (not dimethylammoniumylidene; not dimethylimmonio)
Page 1464, appendix 2, column 2, structure 3. [corrected 17 October 2018]
For (HO)2P(O)–
read (HO)2P–
Page 1464, appendix 2, column 2, structure 4. [corrected 17 October 2018]
For (HO)2P(O)–
read (HO)2P–
Page 1464, appendix 2, column 2, structure 20.
For (CH3)3-C-O–
read (CH3)3C-O–
Page 1465, appendix 2, line 1. [modified 3 March 2021]
For dimethyliminio: see dimethylazaniumylidene*
read dimethylimmonio: see N-methylmethanaminiumylidene*
Page 1466, appendix 2, line 19. [corrected 8 May 2019]
For disiloxan-1-yl*
read disiloxanyl*
Page 1466, Appendix 2, lines 24/25. [modified 22 January 2020]
For (disulfanylcarbonyl)oxy = (disulfanecarbonyl)oxy* = (dithiohydroperoxycarbonyl)oxy
read (disulfanylcarbonyl)oxy* = [(dithiohydroperoxy)carbonyl]oxy
Page 1467, Appendix 2, lines 11/12. [modified 22 January 2020]
For [(dithiohydroperoxy)carbonyl]oxy = (disulfanecarbonyl)oxy* = (disulfanylcarbonyl)oxy
read [(dithiohydroperoxy)carbonyl]oxy = (disulfanylcarbonyl)oxy*
Page 1467, appendix 2, lines 14/15. (modified 27 November 2019)
For 1,2-dithiooxalyl = ethanebis(thioyl)* = bis(sulfanylidene)ethane-1,2-diyl
read dithiooxalyl = ethanebis(thioyl)* = bis(sulfanylidene)ethanediyl
Page 1468, appendix 2, lines 6/7. (modified 27 November 2019)
For ethanediimidoyl* = oxalimidoyl = 1,2-diiminoethane-1,2-diyl
read ethanediimidoyl* = oxalimidoyl = diiminoethanediyl
Page 1468, appendix 2, lines 8/9. [corrected 8 May 2019]
For ethanedioyl = oxalyl* = 1,2-dioxoethane-1,2-diyl
read ethanedioyl = oxalyl* = dioxoethanediyl
Page 1468, appendix 2, line 17.
For = ethanedioylbis(azanetriyl
read = ethanedioylbis(azanetriyl)
Page 1468, appendix 2, lines 20/21. [modified 20 January 2021]
For ethane-1,2-diylbis(oxy)* = ethylenebis(oxy) (not ethane-1,2-diyldioxy)
read ethane-1,2-diylbis(oxy)* = ethylenebis(oxy) (not ethane-1,2-diyldioxy, not ethylenedioxy)
Page 1468, appendix 2, line 23. [corrected 8 May 2019]
For ethanehydrazonamido* = ethanehydrazonoylamino
read ethanehydrazonamido* = (ethanehydrazonoyl)amino
Page 1468, appendix 2, line 26. [corrected 8 May 2019]
For ethanehydrazonylamino = ethanehydrazonamido*
read (ethanehydrazonyl)amino = ethanehydrazonamido*
Page 1469, appendix 2, lines 6/7. [corrected 15 May 2019]
For ethanethioamido* = ethanethioylamino = thioacetamide
read ethanethioamido* = (ethanethioyl)amino = thioacetamide
Page 1469, appendix 2, lines 10/11. [corrected 15 May 2019]
For ethanethioylamino = ethanethioamido* = thioacetamido
read (ethanethioyl)amino = ethanethioamido* = thioacetamido
Page 1469, appendix 2, line 14.
For ethanimidohydrazido* = 2-ethanimidoylhydrazin-1-yl
read ethanimidohydrazido* = 2-(ethanimidoyl)hydrazin-1-yl
Page 1469, appendix 2, line 17. [corrected 15 May 2019]
For 2-ethanimidoylhydrazin-1-yl = ethanimidohydrazido*
read 2-(ethanimidoyl)hydrazin-1-yl = ethanimidohydrazido*
Page 1470, appendix 2, line 15. [corrected 15 May 2019]
For ethylsulfinimidoyl = ethanesulfinimidoyl*
read S-ethylsulfinimidoyl = ethanesulfinimidoyl*
Page 1470, appendix 2, line 17. [corrected 15 May 2019]
For ethylsulfonimidoyl = ethanesulfonimidoyl*
read S-ethylsulfonimidoyl = ethanesulfonimidoyl*
Page 1471, appendix 2, line 5.
For formohydrazido = 2-formylhydrazin-1-yl*
read formohydrazido* = 2-formylhydrazin-1-yl
Page 1471, appendix 2, line 13.
For 2-formylhydrazin-1-yl* = formohydrazido
read 2-formylhydrazin-1-yl = formohydrazido*
Page 1472, appendix 2, line 1. [corrected 15 May 2019]
For furan-2-ylmethyl* (not furfuryl)
read (furan-2-yl)methyl* (not furfuryl)
Page 1472, appendix 2, line 2. [corrected 15 May 2019]
For furfural (2-isomer only): see furan-2-ylmethyl*
read furfuryl (2-isomer only): see (furan-2-yl))methyl*
Page 1473, appendix 2, line 13. [corrected 15 May 2019]
For heptylidyne* = heptan-1-ylidyne
read heptylidyne* = heptanylidyne
Page 1473, appendix 2, lines 22/23. [modified 30 December 2020]
Delete this correction
Page 1473, appendix 2, lines 24-26. [corrected 15 May 2019]
For hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-tris(sulfanylidenemethylene) = hexane-2,3,5-triyltris(thioxomethylene)
read hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(sulfanylidenemethylene) = hexane-2,3,5-triyltris(thioxomethylene)
Page 1474, appendix 2, above line 1. [corrected 9 December 2020]
Add
| hexane-2,3,5-triyltricarbonyl = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-triyltris(oxomethylene) hexane-2,3,5-triyltris(oxomethylene) = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-tryltricarbonyl | 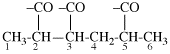 | P-65.1.7.4.2 |
Page 1474, appendix 2, lines 1/2.. [corrected 9 December 2020]
For hexane-2,3,5-tris(sulfanylidenemethylene) = hexane-2,3,5-tricarbothioyl*
read: hexane-2,3,5-triyltris(thioxomethylene) = hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(sulfanylidenemethylene)
hexane-2,3,5-triyltris(sulfanylidenemethylene) = hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(thioxomethylene) [N.B. two entries]
Page 1474, appendix 2, lines 3-5.. [modified 9 December 2020]
Delete these entries
Page 1474, appendix 2, below line 3. [corrected 15 May 2019]
Add hexane-2,3,5-triyltris(oxomethylene) = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-triyltricarbonyl 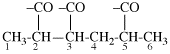 P-65.1.7.4.2
P-65.1.7.4.2
Page 1474, appendix 2, line 14. [corrected 15 May 2019]
For hydrazimidophosphoryl = phosphorohydrazidimidoyl*
read hydrazidimidophosphoryl = phosphorohydrazidimidoyl*
Page 1474, appendix 2, lines 22-24. [corrected 15 May 2019]
For hydrazinecarboximidoyl* = hydrazinyl(imino)methyl = C-hydrazinylcarbonimidoyl = carbonohydrazimidoyl
read hydrazinecarboximidoyl* = hydrazinyl(imino)methyl = C-hydrazinylcarbonimidoyl = carbonohydrazidimidoyl
Page 1474, appendix 2, column 3 line 7.
For P-29.
read P-29.3.2.1; P-29.3.2.2
Page 1475, appendix 2, line 13.
For hydraziny(lhydrazinylidene)methyl
read hydrazinyl(hydrazinylidene)methyl
Page 1475, appendix 2, line 22. [corrected 28 October 2016]
For hydrazinylidene* = diazenylidene
read hydrazinylidene* = diazanylidene
Page 1475, appendix 2, line 24. [corrected 15 May 2019]
Delete hydrazinylidene(amino)methyl = carbamohydrazonyl* H2N-C(=NH-NH2)– P-66.4.2.3
Page 1476, appendix 2, lines 5/6. [modified 10 February 2021]
For hydrazinylidenemethylidene* = diazenylidenemethylidene = hydrazonomethylidene
read hydrazinylidenemethylidene* = diazanylidenemethylidene (not hydrazonomethylidene)
Page 1476, appendix 2, lines 22/23. [corrected 22 May 2019]
For hydroperoxyphosphoryl = phosphoroperoxoyl* = peroxyphosphoryl
read (hydroperoxy)phosphoryl = phosphoroperoxoyl* = peroxyphosphoryl
Page 1476, appendix 2, line 26. [corrected 22 May 2019]
For hydroselenonyl*
read hydroseleninyl* [move to above the previous entry]
Page 1476, appendix 2, column 2, structure 6.
For H2-N-NH-SO2–
read H2N-NH-SO2–
Page 1477, appendix 2, line 18. [corrected 22 May 2019]
For (C-hydroxycarbonimidoyl)amino* = [hydroxyl(imino)methyl]amino
read (C-hydroxycarbonimidoyl)amino* = [hydroxy(imino)methyl]amino
Page 1478, appendix 2, line 1. [corrected 22 May 2019]
For hydroxyl(mercapto)phosphoryl: see hydroxy(sulfanyl)phosphoryl*
read hydroxy(mercapto)phosphoryl: see hydroxy(sulfanyl)phosphoryl*
Page 1478, appendix 2, ccolumn 2, structure 16.
For (HO)(HS)B)–
read (HO)(HS)B–
Page 1479, appendix 2, line 6. [corrected 22 May 2019]
For imino* (not azanylidene)
read imino* = azanylidene (see also azanediyl*)
Page 1480, appendix 2, column 2, line 5.
For (CH3)2CH-O
read (CH3)2CH-O–
Page 1481, appendix 2, line 1. (corrected 4 December 2019)
For isothiocyanatosulfonyl* = sulfurisothiocyanatidoyl
read isothiocyanatosulfonyl* = sulfur(isothiocyanatidoyl)
Page 1481, appendix 2, line 3.
For 3-isouriedo: see [(aminohydroxy)methyidene]amino*
read 3-isoureido: see [amino(hydroxy)methylidene]amino*
Page 1483, appendix 2, line 17.
For S-methoxycarbonimidoyl*
read C-methoxycarbonimidoyl*
Page 1484, appendix 2, line 18. [corrected 29 May 2019]
For methylenebis(thio) = methylenebis(sulfanyl)*
read methylenebis(thio) = methylenebis(sulfanediyl)*
Page 1484, appendix 2, lines 28-29 and move after line 23. [modified 9 December 2020]
For (1-methylethyl)oxy = propan-2-yloxy* = isopropoxy
read 1-methylethoxy = (propan-2-yl)oxy* = isopropoxy
Page 1484, appendix 2, column 2, structure 16. [corrected 29 May 2019]
For –CH2-C(CH3)–
read –CH2-CH(CH3)–
Page 1485, appendix 2, line 7. (corrected 4 December 2019)
For 2-methylphenyl* = o-tolyl
read 2-methylphenyl* = o-tolyl
Page 1485, appendix 2, column 2, line 10.
For CH3-C+-(CH3)-CH2–
read CH3-C+(CH3)-CH2–
Page 1488, appendix 2, line 1. [modified 9 December 2020]
Move to before the last entry on the previous page.
for 2-naphthyl = naphthalene-2-yl*
read 2-naphthyl = naphthalen-2-yl*
Page 1488, appendix 2, line 13. [modified 9 December 2020]
For nitroamino: see nitramido*
read nitroamino = nitramido* O2N-NH– P-67.1.4.3.2
Page 1488, appendix 2, line 15.
For 1-nitrohydrazin_1-yl*
read 1-nitrohydrazin-1-yl*
Page 1489, appendix 2, line 15.
For CH3-[CH2]6-C≡
read CH3-[CH2]6-CH2–
Page 1489, appendix 2, column 2, structure 10.
Replace structure with:
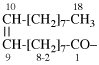
Page 1489, appendix 2, column 2, structure 19.
Replace structure with:
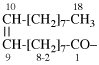
Page 1489, appendix 2, last line. [corrected 18 September 2019]
Delete this line
Page 1490, appendix 2, line 3-4. [modified 29 May 2019]
For oxalo* = not carboxycarbonyl; not carboxyformyl; not hydroxyl(oxo)acetyl)]
read oxalo* = carboxycarbonyl [not carboxyformyl; not hydroxy(oxo)acetyl]
Page 1490, appendix 2, line 7. [modified 29 May 2019]
For oxalooxy* = (carboxyczrbonyl)oxy
read oxalooxy* = (carboxycarbonyl)oxy [not (carboxyformyl)oxy]
Page 1490, appendix 2, line 10.
For oxalylbis(azanediyl)* = ethanedioybis(azanediyl)
read oxalylbis(azanediyl)* = ethanedioylbis(azanediyl)
Page 1490, appendix 2, line 25.
For oxidanyl = hydroxy* HO– P-63.1.4
read oxidanyl: see hydroxy*
Page 1490, appendix 2, line 28. [corrected 18 September 2019]
For oxoacetyl* = oxaldehydoyl
read oxoacetyl*
Page 1490, appendix 2, column 2, structure 8.
Replace structure with:
>N-CO-CO-N<
Page 1490, appendix 2, line 28. [corrected 18 September 2019]
For oxoacetyl* = oxaldehydoyl
read oxoacetyl*
Page 1491, appendix 2, column 2, structure 13.
Replace structure with:
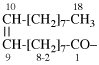
Page 1492, appendix 2, line 9. [modified 29 May 2019]
For 2-oxopropylidene* (not acetonylidene)
read 2-oxopropylidene* (not acetonylidene) CH3-CO-CH= P-64.5
Page 1492, appendix 2, line 14. [modified 21 April 2021]
Delete this line oxyl* = ylooxidanyl
Page 1492, appendix 2, column 2, line 6.
Replace structure with:
CH3-CO-CH2–
Page 1492, appendix 2, column 2, line 22.
For P-29.3.2.1; P-20.3.2.2
read P-29.3.2.1; P-29.3.2.2
Page 1493, appendix 2, line 24. [modified 30 December 2020]
For phenylcarbonyl: see benzoyl*
read phenylcarbonyl = benzoyl* = benzenecarbonyl = oxo(phenyl)methyl C6H5-CO– P-34.2.1.1; P-34.2.2; P-65.1.7.2.1
Page 1495, appendix 2, line 3.
For = 14-phenylenebis(oxomethylene)
read = 1,4-phenylenebis(oxomethylene)
Page 1495, appendix 2, column 2, structure 17.
For C6H5-Se-O–
read C6H5-S-O–
Page 1497, appendix 2, lines 12-14. Correction deleted [11 November 2020]
Page 1499, appendix 2, column 2, structure 3.
For CH3-CH3-CS–
read CH3-CH2-CS–
Page 1499, appendix 2, column 2, structure 10. [corrected 5 June 2019]
For CH3-CH2-C=
read CH3-CH2-CH=
Page 1502, appendix 2, line 21.
For selenocyanato* = carbononitridoylsylfanyl
read selenocyanato* = carbononitridoylselanyl
Page 1503, appendix 2, line 4. (modified 27 November 2019)
For silanediyldiethane-1,2-diyl* = silanediyldiethylene
read silanediyldi(ethane-2,1-diyl)* = silanediyldiethylene
Page 1503, appendix 2, line 5 [modified 22 January 2020]
For silanediyldiethylene = silanediyldiethane-1,2-diyl*
read silanediyldiethylene = silanediyldi(ethane-2,1-diyl)*
Page 1503, appendix 2, line 10.
For P-29.3.1; 2; P-68.2.2
read P-29.3.1; P-68.2.2
Page 1504, appendix 2, column 2, structure 17.
For (HO)2Sb(O)—
read (HO)2Sb(O)–
Page 1505, appendix 2, lines 16-18. [modified 7 August 2019]
For 2-sulfanyl-1,2-bis(sulfanylidene)ethyl* = 2-sulfanyl-2-sulfanylideneethanethioyl = trithiooxalo HS-CS-CS– P-65.1.7.2.4
read sulfanylbis(sulfanylidene)ethyl = sulfanyl(sulfanylidene)ethanethioyl* = trithiooxalo HS-CS-CS– P-65.1.7.2.4; P-65.1.7.3.3
Page 1506, appendix 2, lines17-19. [corrected 12 June 2019]
For 2-sulfanyl-1,2-sulfanylideneethanethioyl = 2-sulfanyl-1,2-bis(sulfanylidene)ethyl* = trithiooxalo HS-CS-CS– P-65.1.7.2.4
read sulfanyl(sulfanylidene)ethanethioyl* = sulfanylbis(sulfanylidene)ethyl = trithiooxalo HS-CS-CS– P-65.1.7.2.4; P-65.1.7.3.3
Page 1508, appendix 2, line 2 (modified 4 December 2019)
For sulfurisothiocyantidoyl = isothiocyanatosulfonyl*
read sulfur(isothiocyanatidoyl) = isothiocyanatosulfonyl*
Page 1508, appendix 2, column 3, line 11.
For P-65.3.2.3; P-67-1.4.4.1
read P-65.3.2.3; P-67.1.4.4.1
Page 1508, appendix 2, line 28. [corrected 19 June 2019]
For tetraazanyl*
read tetraazan-1-yl*
Page 1509, appendix 2, line 1.
For –S-S-S–
read –S-S-S-S–
Page 1509, appendix 2, lines 13/14. [corrected 19 June 2019]
For thioacetamido = ethanethioamido* = ethanethioylamino
read thioacetamido = ethanethioamido* = (ethanethioyl)amino
Page 1509, appendix 2, lines 18/19. [corrected 19 June 2019]
For thiobenzamido = benzenecarbothioamido* = benzenecarbothioylamino
read thiobenzamido = benzenecarbothioamido* = (benzenecarbothioyl)amino
Page 1509, appendix 2, column 2, structure 7.
For CH3-CO-NH–
read CH3-CS-NH–
Page 1510, appendix 2, line 1. [modified 19 June 2019]
For thioborono* = hydroxyl(sulfanyl)boranyl
read thioborono* = hydroxy(sulfanyl)boranyl
Page 1510, appendix 2, line 9.
For thiocyanato* = carbonoitridoylsulfanyl
read thiocyanato* = carbononitridoylsulfanyl
Page 1510, appendix 2, column 2, line 1.
For (HO)(HS)B)–
read (HO)(HS)B–
Page 1511, appendix 2, line 6.
For (thioperoxy)phosphoryl = (phosphorothioperoxoyl)*
read (thioperoxy)phosphoryl = phosphoro(thioperoxoyl)*
Page 1511, Appendix 2, line 10. [corrected 19 June 2019]
For thiophen-2-ylmethyl* = thenyl (2- isomer only)
read (2-thiophen-2-yl)methyl* (not thenyl)
Page 1512, Appendix 2, line 14.
For 2-adamanyl
read 2-adamantyl
Page 1513, appendix 2, line 3. [corrected 26 June 2019]
For trisiliranyl* = cyclotrisilan-1-yl
read trisiliranyl* = cyclotrisilanyl
Page 1513, appendix 2, lines 8/9. [modified 7 August 2019]
For trithiooxalo = 2-sulfanyl-2-sulfanylideneethanethioyl* = 2-sulfanyl-1,2-bis(sulfanylidene)ethyl
read trithiooxalo = sulfanyl(sulfanylidene)ethanethioyl* = sulfanylbis(sulfanylidene)ethyl
Page 1513, appendix 2, column 2, structure 14. [corrected 26 June 2019]
For CH2=CH=
read CH2=C=
Page 1514, appendix 2, line 1. [corrected 21 April 2021]
For ylohydroxy: see oxyl*
read ylohydroxy: see ylooxidanyl*
Page 1514, appendix 2, line 3. [corrected 21 April 2021]
For ylooxidanyl = oxyl* (not ylohydroxy)
read ylooxidanyl* = ylooxy (not ylohydroxy)
Page 1517, Appendix 3, structure 6 on this page. [corrected 17 October 2018]
Replace the structure with:
Page 1518, Appendix 3, structure 2 on this page. [corrected 26 September 2018]
Replace the structure with:
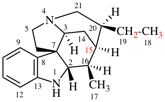
Page 1518, Appendix 3, structure 3 on this page. [corrected 17 October 2018]
Replace the structure with:
Page 1521, Appendix 3, structure 6 on this page. [corrected 17 October 2018]
Replace the structure with:
Page 1522, Appendix 3, ormosanine. [modified 14 October 2020]
For omosanine
read ormosanine**
Page 1522, Appendix 3, structure 5 on this page. [corrected 17 October 2018]
Replace the structure with:
Page 1523, Appendix 3, structure 1 on this page. [corrected 24 October 2018]
Replace the structure with:
Page 1523, appendix 3, structure 2 on this page.
For pancrasine read pancracine
Page 1524, Appendix 3.
For solanidine
read solanidane
replace the structure with:
Page 1524, Appendix 3, spirosolane. [modified 14 October 2020]
For spirosolane**
read spirosolane††
add footnote †† The CAS name for this structure requires the chirality at C-22 to be specified.
Page 1525, Appendix 3, tropane. [modified 14 October 2020]
For 1αH,5αH-tropane**
read tropane**
Page 1525, Appendix 3, structure 4 on this page. [corrected 17 October 2018]
For tubocuran read tubocuraran
replace the structure with:
Page 1526, Appendix 3, structure 4 on this page. [corrected 17 October 2018]
Replace the structure with:
Page 1526, Appendix 3, vobasan. [modified 14 October 2020]
replace the structure with:
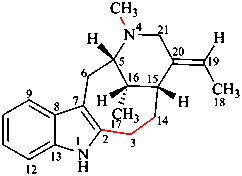
Page 1527, Appendix 3, structure 1 on this page. [corrected 17 October 2018]
Replace the structure with:
.
Page 1528, appendix 3, structure 1 on this page.
Replace structures with:
Page 1529, appendix 3, structure 2 on this page.
Replace structures with:
Page 1529, appendix 3, structure 4 on this page.
For furostane read furostan
Page 1530, appendix 3, structure 2 on this page.
Replace structures with:
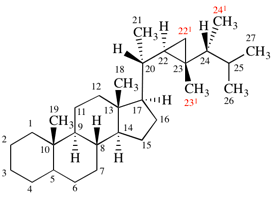
Page 1530, appendix 3, structure 3 on this page.
Replace structures with:
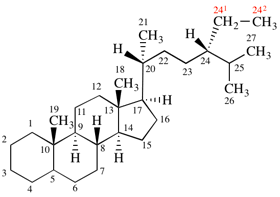
Page 1531, Appendix 3, structure 1 on this page. [modified 1 January 2019]
Replace the structure with:
Page 1531, appendix 3, structure 2 on this page.
Replace structures with:
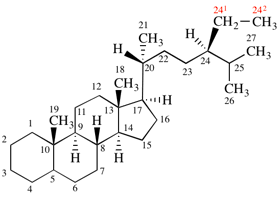
Page 1531, Appendix 3, structure 3 on this page. [corrected 9 January 2019]
Replace structures with:
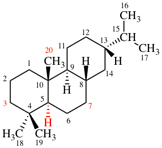
Page 1532, appendix 3, structure 3 on this page. [modified 14 October 2020]
For beyerance**
read beyerane††
Add footnote †† The CAS name for this structure is based on kaurane
Page 1533, appendix 3, example 1 on this page.
For β,![]() -carotene read β,
-carotene read β,![]() -carotene
-carotene
Page 1533, appendix 3, example 3 on this page.
For ε,κ-xarotene
read ε,κ-carotene
Page 1533, appendix 3, example 4 on this page.
For ε,χ-xarotene
read ε,χ-carotene
Page 1536, appendix 3, footnote ††
For The CAS name for this structure is based on gammacercane.
read The CAS name for this structure is based on gammacerane.
Page 1538, Appendix 3.
Replace the structure of oleanane with:

Page 1539, Appendix 3, structure 2 on this page. [corrected 24 October 2018]
Replace the structure with:
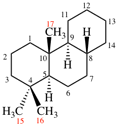
Page 1539, second footnote.
For dammerane read dammarane
Page 1540, Appendix 3, structure 4 on this page. [corrected 17 October 2018]
Replace the structure with:
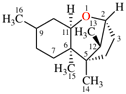
Page 1540, Appendix 3, structure 5 on this page. [corrected 17 October 2018]
Replace the structure with:
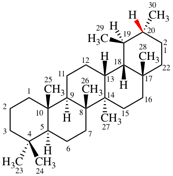
Page 1552, index.
For heterocycls
read heterocycles