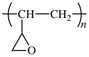
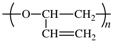
Members of Working Party R. C. Hiorns (France), R. J. Boucher (UK), R. Duhlev (UK), K.-H. Hellwich (Germany), P. Hodge (UK), A. D. Jenkins (UK), R. G. Jones (UK), J. Kahovec (Czech Republic), G. Moad (Australia), C. K. Ober (USA), D. W. Smith (USA), R. F. T. Stepto (UK), J.-P. Vairon (France), and J. Vohlídal (Czech Republic)
This version is as close as possible to the published version [see Pure Appl. Chem. 84(10), 2167-2169 (2012). Copyright IUPAC; reproduced with the permission of IUPAC]. If you need to cite these rules please quote this reference as their source.
A PDF of the printed version is available. Also published in Chem. Int., 2012, 34(6), attached to back cover; Colloid Polym. Sci., 2013, 291, 457-458; Eur. Polym. J., 2013 49, 10-11; Polymer, 2013, 54, 3-4; Polym. Adv. Technol., 2013, 24, i-ii; Polym. Degrad. Stab, 2013, 98, 1-2; Polym. Int., 2013, 62, I-II; Polym. Test., 2013, 32, iv-v; Prog. Polym. Sci., 2013, 38, iii-iv; React. Funct. Polym., 2013, 73, iv-v; Synth. Met., 2013, 1633, vi-vii.
A PDF of the document in Basque versionContents
A PDF of the document in Catalan version
Kem. Ind. 2016, 65, 153-160 (in Croatian))
A PDF of the Galician version
ChemZi, 2020, 16, 32-33 (in Slovak)
A PDF of the Spanish version
- 1. Introduction
- 2.. Basic Concepts
- 3. Source-Based Nomenclature
- 3.1. Homopolymers
- 3.2. Copolymers
- 4. Structure-Based Nomenclature
- 4.1. Regular single-strand organic polymers
- 4.2. Regular double-strand organic polymers
- 5. Nomenclature of Inorganic and Inorganic–Organic Polymers
- 6. Traditional Names
- 7. Graphical Representations
- 8. CA Index Names
- References
Polymer nomenclature usually applies to idealised representations; minor structural irregularities are ignored. A polymer can be named in one of two ways. Source-based nomenclature can be used when the monomer can be identified. Alternatively, more explicit structure-based nomenclature can be used when the polymer structure is proven. Where there is no confusion, some traditional names are also acceptable.
Whatever method is used, all polymer names have the prefix poly, followed by enclosing marks around the rest of the name. The marks are used in the order: {[( )]}. Locants indicate the position of structural features, e.g., poly(4-chlorostyrene). If a source-based name is one word and has no locants, then the enclosing marks are not essential, but they should be used when there might be confusion, e.g., poly(chlorostyrene) is a polymer whereas polychlorostyrene might be a small, multi-substituted molecule. End-groups are described with α- and ω-, e.g., α-chloro-ω-hydroxy-polystyrene.[3]
3. Source-Based Nomenclature [5]
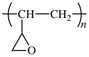

| Copolymer | Qualifier | Example | |
| unspecified | co | (C) | poly(styrene-co-isoprene) |
| statistical | stat | (C) | poly[isoprene-stat-(methyl methacrylate)] |
| random | ran | (C) | poly[(methyl methacrylate)-ran-(butyl acrylate)] |
| alternating | alt | (C) | poly[styrene-alt-(maleic anhydride)] |
| periodic | per | (C) | poly[styrene-per-isoprene-per-(4-vinylpyridine)] |
| block | block | (C) | poly(buta-1,3-diene)-block-poly(ethene-co-propene) |
| graft* | graft | (C) | polystyrene-graft-poly(ethylene oxide) |
| * The first name is that of the main chain. | |||
| (Co)polymer | Qualifier | Example | ||
| blend | blend | (C) | poly(3-hexylthiophene)-blend-polystyrene | |
| comb | comb | (C) | polystyrene-comb-polyisoprene | |
| complex | compl | (C) | poly(2,3-dihydrothieno[3,4-b][1,4]dioxine)-compl-poly(vinylbenzenesulfonic acid)* | |
| cyclic | cyclo | (P) | cyclo-polystyrene-graft-polyethylene | |
| branch | branch | (P) | branch-poly[(1,4-divinylbenzene)-stat-styrene] | |
| network | net | (C or P) | net-poly(phenol-co-formaldehyde) | |
| interpenetrating network | ipn | (C) | (net-polystyrene)-ipn-[net-poly(methyl acrylate)] | |
| semi-interpenetrating network | sipn | (C) | (net-polystyrene)-sipn-polyisoprene | |
| star | star | (P) | star-polyisoprene | |
| * In accordance with IUPAC organic nomenclature, square brackets enclose locants that refer to the numbering of the components of the fused ring. | ||||
(i) a large enough part of the polymer chain is drawn to show the structural repetition, e.g.,
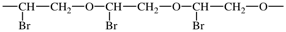
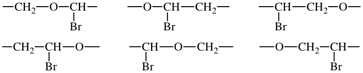
(iv) using the shortest path from the most senior subunit to the next senior, the correct order of the subunits is determined using Fig. 1;
(v) the preferred CRU is chosen as that with the lowest possible locant(s) for substituents.
In the above example, the oxy subunits in the CRUs are heteroatom chains. From Fig. 1, oxy subunits are senior to the acyclic carbon chain subunits, the largest of which are bromo-substituted -CH2-CH2- subunits. 1-Bromoethane-1,2-diyl is chosen in preference to 2-bromoethane-1,2-diyl as the former has a lower locant for the bromo-substituent. The preferred CRU is therefore oxy(1-bromoethane-1,2-diyl) and the polymer is thus named poly[oxy(1-bromoethane-1,2-diyl)]. Please note the enclosing marks around the subunit carrying the substituent.
Polymers that are not made up of regular repetitions of a single CRU are called irregular polymers. For these, each constitutional unit (CU) is separated by a slash, e.g., poly(but-1-ene-1,4-diyl/1-vinylethane-1,2-diyl). [9]
| Name | Group* | Name | Group* |
| oxy | ‐O– | propylimino |  |
| sulfanediyl | ‐S– | hydrazine-1,2-diyl | 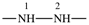 |
| sulfonyl | ‐SO2– | phthaloyl | 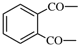 |
| diazenediyl | ‐N=N– | 1,4-phenylene | 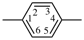 |
| imino | ‐NH– | cyclohexane-1,2-diyl | 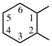 |
| carbonyl | 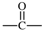 | butane-1,4-diyl | 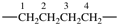 |
| oxalyl | 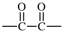 | 1-bromoethane-1,2-diy | 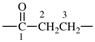 |
| silanediyl | ‐SiH2– | 1-oxopropane-1,3-diyl | 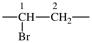 |
| ethane-1,2-diyl | 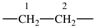 | ethene-1,2-diyl | 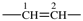 |
| methylene | ‐CH2– | methylmethylene | 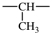 |
| * To avoid ambiguity, wavy lines drawn perpendicular to the free bond, which are conventionally used to indicate free valences, [13] are usually omitted from graphical representations in a polymer context. | |||
Double-strand polymers consist of uninterrupted chains of rings. In a spiro polymer, each ring has one atom in common with adjacent rings. In a ladder polymer, adjacent rings have two or more atoms in common. To iden- tify the preferred CRU, the chain is broken so that the senior ring is retained with the maximum number of heteroatoms and the minimum number of free valences.
An example is 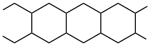 . The preferred CRU is an acyclic subunit of 4 carbon atoms with 4 free valences, one at each atom, as shown below. It is ori- ented so that the lower left atom has the lowest number. The free-valence locants are written before the suffix, and they are cited clockwise from the lower left position as: lower-left, upper-left:upper-right, lower- right. This example is thus named poly(butane-1,4:3,2-tetrayl). For more complex structures, the order of seniority again follows Fig. 1.
. The preferred CRU is an acyclic subunit of 4 carbon atoms with 4 free valences, one at each atom, as shown below. It is ori- ented so that the lower left atom has the lowest number. The free-valence locants are written before the suffix, and they are cited clockwise from the lower left position as: lower-left, upper-left:upper-right, lower- right. This example is thus named poly(butane-1,4:3,2-tetrayl). For more complex structures, the order of seniority again follows Fig. 1.
5. Nomenclature of Inorganic and Inorganic–Organic Polymers [11]
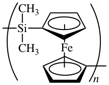
Some regular single-strand inorganic polymers can be named like organic polymers using the rules given above, e.g., -[O-Si(CH3)2]n- and -[Sn(CH3)2]n- are named poly[oxy(dimethylsilanediyl)] and poly(dimethylstannanediyl), respectively. Inorganic polymers can also be named in accordance with inorganic nomenclature, but it should be noted that the seniority of the elements is different to that in organic nomenclature. However, certain inorganic–organic polymers, for example those containing metallocene derivatives, are at present best named using organic nomenclature, e.g., the polymer below can be named poly[(dimethylsilanediyl)ferrocene-1,1′-diyl].
When they fit into the general pattern of systematic nomenclature, some traditional and trivial names for polymers in common usage, such as polyethylene, polypropylene, and polystyrene, are retained.
7. Graphical Representations [12,13]
The bonds between atoms can be omitted, but dashes should be drawn for chain-ends. The seniority of the subunits does not need to be followed. For single-strand (co)polymers, a dash is drawn through the enclosing marks, e.g., poly[oxy(ethane-1,2-diyl)] shown below left. For irregular polymers, the CUs are separated by slashes, and the dashes are drawn inside the enclosing marks. End-groups are connected using additional dashes out- side of the enclosing marks, e.g., α-methyl-ω-hydroxy-poly[oxirane-co-(methyloxirane)], shown below right.
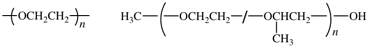
CAS maintains a registry of substances. In the CAS system, the CRU is called a structural repeating unit (SRU). There are minor differences in the placements of locants, e.g., poly(pyridine-3,5-diylthiophene-2,5-diyl) is poly(3,5-pyridinediyl-2,5-thiophenediyl) in the CAS registry, but other- wise polymers are named using similar methods to those of IUPAC.[14,15]
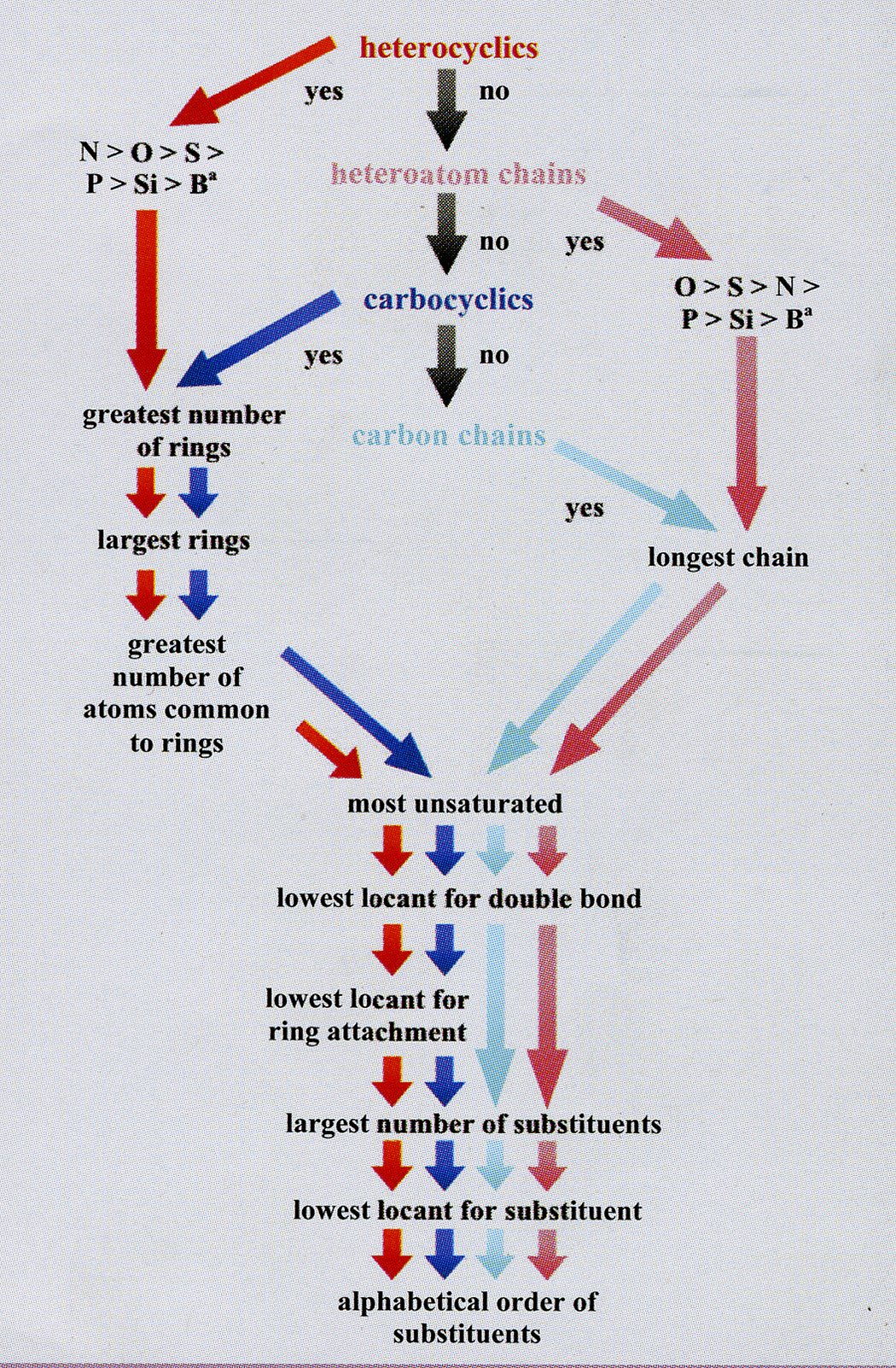
Fig. 1 The order of subunit seniority. The senior subunit is at the top centre. Subunits of lower seniority are found by following the arrows.
The type of subunit, be it a heterocycle, a heteroatom chain, a carbocycle, or a carbon chain, determines the colour of the arrow to follow.
a Other heteroatoms may be placed in these orders as indicated by their positions in the periodic table.[8]
1. Freely available on: (a) https://iupac.org/what-we-do/journals/pure-and-applied-chemistry/; (b) https://iupac.qmul.ac.uk/
2. http://www.cas.org/
3. IUPAC. The “Purple Book”, RSC Publishing, Cambridge, UK (2008).
4. IUPAC. Pure Appl. Chem. 81, 351 (2009).
5. IUPAC. Pure Appl. Chem. 69, 2511 (1997).
6. IUPAC. Pure Appl. Chem. 73, 1511 (2001).
7. IUPAC. Pure Appl. Chem. 57, 1427 (1985).
8. IUPAC. Pure Appl. Chem. 74, 1921 (2002).
9. IUPAC. Pure Appl. Chem. 66, 873 (1994).
10. IUPAC. Pure Appl. Chem. 65, 1561 (1993).
11. IUPAC. Pure Appl. Chem. 57, 149 (1985).
12. IUPAC. Pure Appl. Chem. 66, 2469 (1994).
13. IUPAC. Pure Appl. Chem. 80, 277 (2008).
14. Macromolecules 1, 193 (1968).
15. Polym. Prepr. 41(1), 6a (2000).