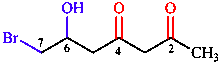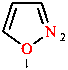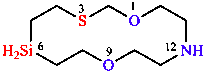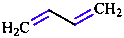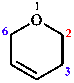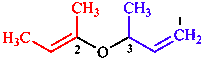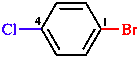IUPAC Division of Chemical Nomenclature and Structure Representation.
Brief Guide to the Nomenclature of Organic Chemistry
IUPAC Technical Report
(version 1.1)
https://iupac.qmul.ac.uk/BriefGuide/organic.html
Members of Working Party (1998-2002): Karl-Heinz Hellwich* (Germany), Richard M. Hartshorn (New Zealand), Andrey Yerin (Russia), Ture Damhus (Denmark) and Alan T. Hutton (South Africa)
World Wide Web version prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London, Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
This version is as close as possible to the published version [see Pure Appl. Chem. 92(3), 527-539 (2020). Copyright IUPAC; reproduced with the permission of IUPAC]. If you need to cite these rules please quote this reference as their source.
A PDF of the printed version is available. An A4 four sided version 1.1 is available (512 KB)
A PDF of the document in Basque version
A PDF of the document in Catalan version
A PDF of the Galician version
ChemZi, 2020, 16, 28-31 (in Slovenian)
A PDF of the Spanish version
Changes from the printed document have been marked by  which is a link to details of the change and where it applies.
which is a link to details of the change and where it applies.
Abstract: This IUPAC Technical Report is one of a series that seeks to distil the essentials of IUPAC nomenclature recommendations. The present report provides a succinct summary of material presented in the publication Nomenclature of Organic Chemistry — IUPAC Recommendations and Preferred Names 2013. The content of this report will be republished and disseminated as a four-sided lift-out document (see supplementary information) which will be available for inclusion in textbooks and similar publications.
Contents
The universal adoption of an agreed nomenclature is a key tool for efficient communication in the chemical sciences, in industry, and for regulations associated with import/export or health and safety. The International Union of Pure and Applied Chemistry (IUPAC) provides recommendations on many aspects of nomenclature [1]. The basics of organic nomenclature are summarized here. There are companion docu- ments on the nomenclature of inorganic [2] and polymer [3] chemistry, with hyperlinks to original docu- ments. An overall summary of chemical nomenclature can be found in Principles of Chemical Nomenclature [4]. Comprehensive detail can be found in Nomenclature of Organic Chemistry, colloquially known as the Blue Book [5], and in the related publications for inorganic compounds (the Red Book) [6], and polymers (the Purple Book) [7].
It should be noted that many compounds may have non-systematic or semi-systematic names and IUPAC rules also allow for more than one systematic name in many cases. Some traditional names (e.g. styrene, urea) are also used within systematic nomenclature. The new edition of the Blue Book [5] incorporates a hierarchical set of criteria for choosing the single name which is to be preferred for regulatory purposes, the Preferred IUPAC Name, or PIN.
Substitutive nomenclature is the main method for naming organic-chemical compounds. It is used mainly for compounds of carbon and elements of Groups 13–17. For naming purposes, a chemical compound is treated as a combination of a parent compound (Section 5) and characteristic (functional) groups, one of which is designated the principal characteristic group (Section 4). A systematic name is based on the name of the most senior parent compound (Section 6) in which the substitution of hydrogen atoms is represented by a suffix for the principal characteristic group(s), prefixes representing less senior characteristic groups and other substituent groups, and locants that specify their locations. Names created according to substitutive nomenclature may also include fragments named in accordance with other nomenclature types or opera- tions. For example, addition and subtraction operations (Section 5.4) are performed mainly to define the hydrogenation state, while a replacement operation defines a replacement of (in most cases) carbon atoms with heteroatoms.
The most common components of a substitutive chemical name are illustrated with reference to the chemical structure shown in Table 1, along with its systematic name and the components of the name.
Locants indicate the position of substituents or other structural features. They are generally placed before the part of the name that indicates the corresponding structural feature. Three kinds of enclosing mark are used, in the nesting order {[( )]}, when it is necessary to indicate which parts of a name belong together.
Multiplicative prefixes (Table 2) are used when more than one fragment of a particular kind is present in a structure. Which kind of multiplicative prefix is used depends on the complexity of the corresponding fragment – e.g. trichloro, but tris(chloromethyl).
Table 1: Components of the substitutive name (4S,5E)-4,6-dichlorohept-5-en-2-one for
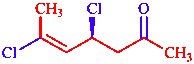
| hept(a) | parent (heptane) | one | suffix for principal characteristic group |
| en(e) | unsaturation ending | chloro | substituent prefix |
| di | multiplicative prefix | E S | stereodescriptors |
| 2 4 5 6 | locants | ( ) | enclosing marks |
Table 2: Multiplicative prefixes for simple and complicated entities.
| No. | Simple | Complicated | No. | Simple | Complicated |
| 2 | di | bis | 8 | octa | octakis |
| 3 | tri | tris | 9 | nona | nonakis |
| 4 | tetra | tetrakis | 10 | deca | decakis |
| 5 | penta | pentakis | 11 | undeca | undecakis |
| 6 | hexa | hexakis | 12 | dodeca | dodecakis |
| 7 | hepta | heptakis | 20 | icosa | icosakis |
The formation of a systematic name requires several steps, to be taken (when they are applicable) in the following order:
a. Determine the principal characteristic group to be cited as the suffix (see Section 4).
b. Determine the senior parent amongst those structural components attached to a principal characteristic group (see Sections 5 and 6).
c. Name the parent hydride and specify any unsaturation (Section 5).
d. Combine the name of the parent hydride with the suffix for the principal characteristic group (Section 4). e. Identify the substituents and arrange the corresponding prefixes in alphabetical order.
f. Insert multiplicative prefixes, without changing the already established order, and insert locants.
g. Determine chirality centres and other stereogenic units, such as double bonds, and add stereodescriptors.
The presence of a characteristic (or functional) group is denoted by a prefix or suffix attached to the parent name. The names of common characteristic groups are given in Table 3, in order of decreasing seniority. The most senior one, the principal characteristic group, is cited as the suffix, while all other groups are cited as prefixes. Note that, for nomenclature purposes, C–C multiple bonds are not considered to be characteristic groups (Section 5.4).
Depending on the number and arrangement of carbon-containing suffix groups, the carbon atom can be a part of the parent compound (e.g. –(C)OOH, ‘oic acid’) or may be treated as an attachment to a parent compound (e.g. –COOH, ‘carboxylic acid’).
 | 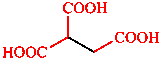 |
| butanedioic acid | ethane-1,1,2-tricarboxylic acid |
Table 3: Seniority order for characteristic groups.
| Class | Formula* | Suffix | Prefix |
| Carboxylates | –COO– | carboxylate | carboxylato |
| –(C)OO– | oate | |
| Carboxylic acids | –COOH | carboxylic acid | carboxy |
| –(C)OOH | oic acid | |
| Esters | –COOR | (R) ...carboxylate** | (R)oxycarbonyl |
| –(C)OOR | (R) ...oate** | |
| Acid halides | –COX | carbonyl halide | halocarbonyl |
| –(C)OX | oyl halide | |
| Amides | –CONH2 | carboxamide | carbamoyl |
| –(C)ONH2 | amide | |
| Nitriles | –C≡N | carbonitrile | cyano |
| –(C)≡N | nitrile | |
| Aldehydes | –CHO | carbaldehyde | formyl |
| –(C)HO | al | oxo |
| Ketones | =O | one | oxo |
| Alcohols | –OH | ol | hydroxy |
| Thiols | –SH | thiol | sulfanyl*** |
| Amines | –NH2 | amine | amino |
| Imines | =NH | imine | imino |
* Here –(C) indicates that the carbon atom is implied by the parent name.
** Here (R) means that the group R is expressed as a separate prefixed word.
*** Note: ‘mercapto’ is no longer acceptable (but is still used by CAS).
 | 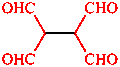 |
| propanedinitrile | ethane-1,1,2,2-tetracarbaldehyde |
Other characteristic groups on a parent compound are represented by appropriate prefixes cited in alphabetical order (here in blue, where R represents an alkyl or aryl group), including also ethers (–OR), (R)oxy; sulfides (–SR), (R)sulfanyl; –Br, bromo; –Cl, chloro; –F, fluoro; –I, iodo; and –NO2, nitro.
 | 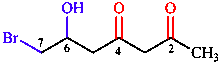 |
| 2-aminoethan-1-ol | 7-bromo-6-hydroxyheptane-2,4-dione |
Several types of parent compounds are used in substitutive nomenclature. Parent compounds without characteristic groups are called parent hydrides. These can be classified as either chains or rings, and may contain carbon atoms and/or heteroatoms. The ring parent compounds can be monocyclic, bridged polycyclic (rings sharing more than two atoms), fused polycyclic (rings sharing two neighbouring atoms), or spiro polycyclic (rings sharing only one atom). More complex parent compounds include bridged fused systems, ring assemblies, cyclophanes, and fullerenes. The atom numbering of a parent compound is defined by the corresponding rules for each type of parent compound. Thereafter, the rules outlined in Section 7 are applied.
The names for saturated carbon chains (alkanes) are composed of the simple numerical term indicating the number of carbon atoms (Table 2, with the ‘a’ elided) together with an ‘ane’ ending (see Table 4), with the exception of the first four alkanes: methane, CH4; ethane, CH3CH3; propane, CH3CH2CH3; butane, CH3[CH2]2CH3.
Table 4: Names for some linear alkanes.
| CH3[CH2]3CH3 | CH3[CH2]7CH3 | CH3[CH2]18CH3 |
| pentane | nonane | icosane |
| CH3[CH]4CH3 | CH3[CH2]16CH3 | CH3[CH2]20CH3 |
| hexane | octadecane | docosane |
The names of saturated carbon monocycles (cycloalkanes) are composed of the prefix ‘cyclo’ and the name of the corresponding alkane.
 |  | 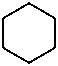 | 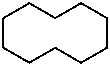 |
| cyclopropane | cyclobutane | cyclohexane | cyclodecane |
A number of non-systematic names have been retained for common rings, for example benzene and the following heterocycles.
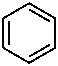 | 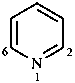 | 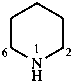 | 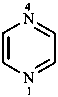 | 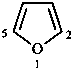 |
| benzene | pyridine | piperidine | pyrazine | furan |
Systematic names for monocycles that contain heteroatoms are constructed in accordance with either the Hantzsch-Widman (H-W) system (3- to 10-membered rings) or replacement nomenclature (larger rings) [4, 5]. Both systems make use of the ‘a’ prefixes shown in Table 5, in which the seniority decreases from left to right across the first row and then the second row.
The H-W system combines the ‘a’ prefixes of Table 5 in decreasing order of seniority with endings, in the H-W system called stems, that indicate the size and saturation of the ring (Table 6). Appropriate locants are added to describe the location of the replacements in the ring and the ‘a’ is elided when followed by a vowel. If there are more than 10 atoms in the ring, replacement nomenclature is used, in which ‘a’ prefixes are again listed in decreasing order of seniority, with locants, before the parent name. The atom numbering is explained in Section 7.
 | 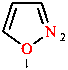 | 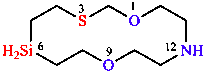 |
| 1,3-dioxane | 1,2-oxazole | 1,9-dioxa-3-thia-12-aza-6-silacyclotetradecane |
Table 5: Selected ‘a’ prefixes for H-W and replacement systems.
| O | oxa | S | thia | N | aza | P | phospha |
| As | arsa | Si | sila | Sn | stanna | B | bora |
Table 6: Stems in the Hantzsch-Widman system. 
| Ring size | Unsaturated | Saturated |
| 3 | irine*/irene | iridine/irane** |
| 4 | ete | etidine/etane** |
| 5 | ole | olidine/olane** |
| 6 | ine/ine/inine*** | ane/inane/inane*** |
| 7 | epine | epane |
* For rings with only N heteroatom(s) ** For rings with/without N heteroatom(s)
*** For O,S/N,Si,Sn/P,As,B as the last cited heteroatom, respectively.
The names of bridged polycyclic systems are based on the name of the alkane with the same number of carbon atoms, which is preceded by an indicator of the number of cycles present and a bridge descriptor that defines the sizes of the various rings; this descriptor gives the number of skeletal atoms in each of the bridges connecting the bridgeheads and is given by arabic numerals cited in descending numerical order, separated by full stops and enclosed in square brackets. Numbering starts at a bridgehead and goes around the rings in order (largest to smallest). Replacement nomenclature (see Section 5.2) is used to name the related heterocycles.
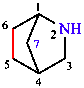 | 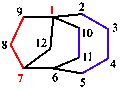 |
| 2-azabicyclo[2.2.1]heptane | tricyclo[4.3.2.11,7]dodecane |
The names of spiro polycyclic systems, in which there is a single atom in common to the rings, include the number of spiro junctions, a bridge descriptor, and the name of the alkane with the same number of carbon atoms. Again, the related heterocycles are named in accordance with replacement nomenclature (see Section 5.2).
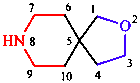 | 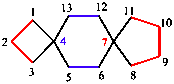 |
| 2-oxa-8-azaspiro[4.5]decane | dispiro[3.2.47.24]tridecane |
Fused polycycles are cyclic systems having one common bond for any pair of adjacent rings.
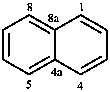 | 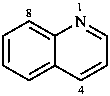 | 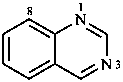 | 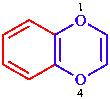 |
| naphthalene | quinoline | quinazoline | 1,4-benzodioxine |
In the systematic nomenclature of fused polycycles, the names for the components are combined and a fusion descriptor indicates how the components are connected. At the end, the structure is renumbered. This process is beyond the scope of the current guide (see ref. [5] for details).
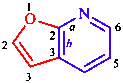 | 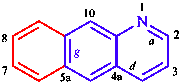 |
| furo[2,3-b]pyridine | benzo[g]quinoline |
The degree of unsaturation of a compound in comparison to a saturated parent can be indicated by replace- ment of the ‘ane’ ending by ‘ene’ and ‘yne’ endings that define the presence of double and triple bonds, respectively, and addition of locants to define their locations.
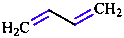 |  |  |
| buta-1,3-diene | pent-1-en-4-yne | cyclohexa-1,3-diene |
The addition of hydrogen to unsaturated parent hydrides is represented by the addition of hydro prefixes to indicate saturation of double bonds, again with locants to define where this occurs.
 | 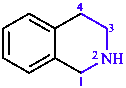 |
| 3,4-dihydropyridine | 1,2,3,4-tetrahydroisoquinoline |
For some unsaturated parent hydrides, the saturated positions are specified using the indicated hydrogen convention
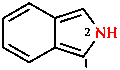 |  | 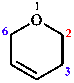 |
| 2H-isoindole | 3H-indole | 3,6-dihydro-2H-pyran |
In cases where a group derived from a parent hydride is a substituent on another parent compound, the substituent name is created by addition of the suffixes ‘yl’ or ‘ylidene’ to the parent hydride name, with the corresponding locants indicating the position of the attachment. The attachment positions expressed by the suffixes ‘yl’ or ‘ylidene’ are senior to any characteristic group (see Section 4, Table 3).
 |  |  |
| butyl | butan-2-ylidene | pyrimidin-5-yl |
A combination of a parent hydride with a functional group may form a functional parent named as single entity. Such names are used as systematic names only if they express the parent compound and the most senior characteristic group of the compound under consideration, e.g. 4-chloroaniline, but 4-aminobenzoic acid (not 4-carboxyaniline or aniline-4-carboxylic acid).
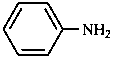 | 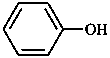 | 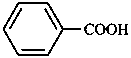 | 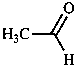 |
| aniline | phenol | benzoic acid | acetaldehyde |
The systematic name is based on the name of the senior parent compound, which is chosen by applying the following criteria in the order described below and shown in Fig. 1, until a decision is reached. For a complete set of criteria see Ref. [8]. In the examples below, the senior parent compound is shown in blue, and a key reason is given alongside.
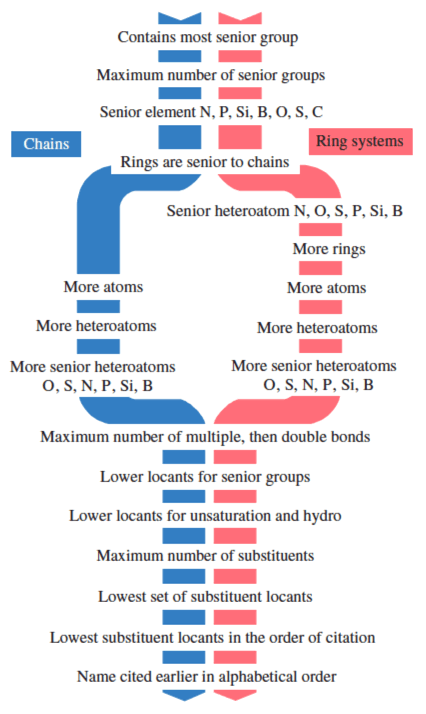
Fig. 1: Criteria for choosing the senior parent compound.
a. Contains the principal characteristic group
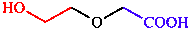 | acid is senior to alcohol |
| (2-hydroxyethoxy)acetic acid | |
b. Maximum number of principal characteristic groups
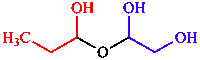 | parent with two characteristic groups is senior |
| 1-(1-hydroxypropoxy)ethane-1,2-diol | |
c. Parent based on senior element (N, P, Si, B, O, S, C)
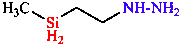 | hydrazine is senior to silane (N senior to Si) |
| [2-(methylsilyl)ethyl]hydrazine | |
d. Rings are senior to chains if composed of the same elements
 | cyclobutane is senior to pentane |
| pentylcyclobutane | |
Note 1: After this criterion, only rings or only chains remain for further choice.
Note 2: In earlier recommendations, seniority depended on number of atoms.
e. Criteria for cyclic systems
e.1. Contains most senior heteroatom in the order N, O, S, P, Si, B.
 | O-ring is senior to P-ring |
| (phosphetan-3-yl)oxirane | |
e.2. Contains more rings
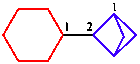 | bicycle is senior to monocycle |
| 2-cyclohexylbicyclo[1.1.1]pentane | |
e.3. Contains more atoms
 | cyclopentane is senior to cyclobutane |
| cyclobutylcyclopentane | |
e.4. Contains more heteroatoms
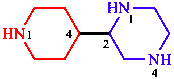 | piperazine, having two heteroatoms, is senior to piperidine |
| 2-(piperidin-4-yl)piperazine | |
e.5. Contains more senior heteroatoms
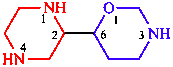 | oxazinane, containing O and N, is senior to piperazine with two N atoms |
| 6-(piperazin-2-yl)-1,3-oxazinane | |
f. Criteria for chains
f.1. Contains more atoms
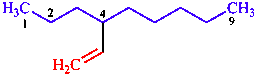 | nine-atom chain is senior to eight-atom chain (even if it has fewer double bonds) |
| 4-ethenylnonane | |
Note: In earlier recommendations unsaturation was senior to chain length.
The following criteria are then applied to chains as well as rings:
g. Contains more multiple, and then double bonds
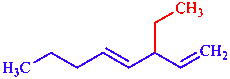 | 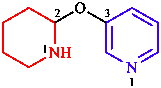 |
| 3-ethylocta-1,4-diene | 3-[(piperidin-2-yl)oxy]pyridine |
h. Having lower locants for principal characteristic groups
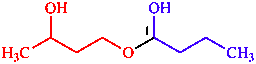 | butan-1-ol is senior to butan-2-ol |
| 1-(3-hydroxybutoxy)butan-1-ol | |
i. Lower locants for unsaturation or hydro prefixes
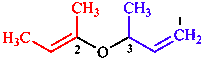 | 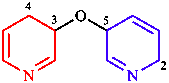 |
| 3-[(but-2-en-2-yl)oxy]but-1-ene | 5-[(3,4-dihydropyridin- 3-yl)oxy]-2,5-dihydropyridine |
j. Maximum number of substituents
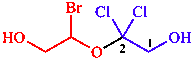 | parent with three substituents is senior to parent with two substituents |
| 2-(1-bromo-2-hydroxyethoxy)-2,2-dichloroethan-1-ol | |
k. Lowest set of locants for all substituents
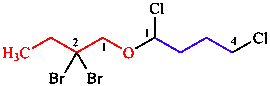 | all parent locants are arranged in increasing order and compared one by one:
1,1,4 is lower than 1,2,2 |
| 1,4-dichloro-1-(2,2-dibromobutoxy)butane | |
Note: Not 2,2-dibromo-1-(1,4-dichlorobutoxy)butane
l. Lowest substituent locants in order of citation
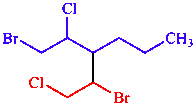 | 1,3,2 is lower than 2,3,1 |
| 1-bromo-3-(1-bromo-2-chloroethyl)-2-chlorohexane | |
Note: Not 2-bromo-3-(2-bromo-1-chloroethyl)-1-chlorohexane
m. Name appearing earlier in alphabetical order
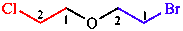 |
| 1-bromo-2-(2-chloroethoxy)ethane |
Note: Not 1-(2-bromoethoxy)-2-chloroethane
The numbering of the parent compound is determined by the compound class and then chosen by consider- ing all possible sets of locants and successively applying the following criteria:
a. Lowest locants for heteroatoms;
b. Lowest locant(s) for indicated hydrogen;
c. Lowest locant(s) for principal characteristic group(s);
d. Lowest locants for ‘ene’, ‘yne’, and hydro prefixes;
e. Lowest locants as a set for all substituents cited by prefixes;
f. Lowest locants for substituents in the order of citation.
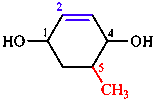 | a. + b. Do not apply;
c. Begin at a C with OH attached;
d. Number towards double bond;
e. Choose numbering that puts CH3 at position 5 rather than position 6. |
| 5-methylcyclohex-2-ene-1,4-diol |
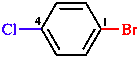 | a. – e. Do not apply;
f. Bromo is cited before chloro in the name and therefore receives the lower locant. |
| 1-bromo-4-chlorobenzene (not 4-bromochlorobenzene) |
| Correct numbering is extremely important, because a single incorrect locant makes it impossible for the reader of the name to work out the correct structure. |
|
Functional class names (formerly radicofunctional names) are preferred for esters and acid halides. For other compound classes (e.g. ethers, ketones, sulfoxides, and sulfones) functional class names are still in use, although substitutive names are preferred. Functional class names consist of one or more substitu- ent names, ordered alphabetically, and followed by the compound class name (separated with spaces as required). Thus CH3C(O)O–CH3 is named methyl acetate, ClCH2C(O)O–CH3 is methyl chloroacetate, CH3C(O)–Cl is acetyl chloride, C6H5C(O)–Br is benzoyl bromide, and (H3C)2SO2 is named dimethyl sulfone.
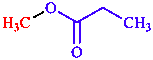 |  |  |
| methyl propanoate | diethyl ether
or ethoxyethane | ethyl methyl ketone
or butan-2-one |
Stereoisomers are differentiated from each other by stereodescriptors cited in names and assigned in accord- ance with the Cahn-Ingold-Prelog (CIP) rules [9, 10]. The most common descriptors are those for the absolute configuration of tetrahedral stereogenic centres (R/S) and those for the configuration of double bonds (E/Z). Locants are added to define the locations of the stereogenic centres and the full set of descriptors is enclosed in parentheses.

(1E,4S,5Z)-1-[(2R)-2-hydroxypropoxy]hepta-1,5-dien-4-ol
Other stereodescriptors (e.g. cis/trans, M/P, C/A) are used in special cases. The non-italic descriptors α/β and D/L (small capitals) are commonly and only used for natural products, amino acids, and carbohydrates.
CAS maintains a registry of chemical substances collected from publications [11]. In the CAS system, com- pounds are named using methods similar to, but not identical with, those of IUPAC. The most prominent difference is the use of ‘CA Index Names’, which in the index are cited in a special inverted order that was devised for the creation of alphabetical indexes of chemical names. CAS also uses conjunctive nomenclature, in which parent compounds are combined to make a new, larger parent compound. In the example below, the conjunctive parent name is benzeneacetic acid (corresponding substitutive name: phenylacetic acid), while the substitutive name recommended by IUPAC for this example is based on the longer chain parent compound propanoic acid.
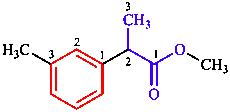
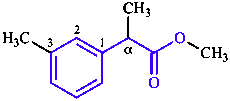
IUPAC name: methyl 2-(3-methylphenyl)propanoate (1)
CA name: methyl α,3-dimethylbenzeneacetate (2)
In index inverted to: benzeneacetic acid, α,3-dimethyl-, methyl ester
Other differences include the position of locants and stereodescriptors, as well as some specific nomenclature procedures.
The structural formulae of organic-chemical compounds are usually drawn in accordance with the zig-zag convention as used widely above [12]. In this convention, all carbon atoms (and their attached hydrogen atoms) attached to at least two other non-hydrogen atoms are represented by the intersection of two lines representing bonds. Hydrogen atoms attached to heteroatoms must not be omitted. In such graphical rep- resentations, each end of a line, each angle, and each intersection represents a carbon atom saturated with hydrogen. Special conventions are used to represent the configuration of stereogenic centres and double bonds [13].
[1] Freely available at: http://www.iupac.org/publications/pac/; or https://iupac.qmul.ac.uk/.
[2] R. M. Hartshorn, K.-H. Hellwich, A. Yerin, T. Damhus, A. T. Hutton. Pure Appl. Chem. 87, 1039 (2015).
[3] R. C. Hiorns, R. J. Boucher, R. Duhlev, K.-H. Hellwich, P. Hodge, A. D. Jenkins, R. G. Jones, J. Kahovec, G. Moad, C. K. Ober, D. W. Smith, R. F. T. Stepto, J.-P. Vairon, J. Vohlídal. Pure Appl. Chem. 84, 2167 (2012).
[4] Principles of Chemical Nomenclature — A Guide to IUPAC Recommendations, 2011 Edition, G. J. Leigh (Ed.), RSC Publishing, Cambridge, U.K. (2011), ISBN 978-1-84973-007–5.
[5] Nomenclature of Organic Chemistry — IUPAC Recommendations and Preferred Names 2013, H. A. Favre, W. H. Powell (Eds.), Royal Society of Chemistry, Cambridge, U.K. (2014), ISBN 978-0-85404-182-4; errata: https://iupac.qmul.ac.uk/bibliog/BBerrors.html.
[6] Nomenclature of Inorganic Chemistry — IUPAC Recommendations 2005, N. G. Connelly, T. Damhus, R. M. Hartshorn, A. T. Hutton (Eds.), RSC Publishing, Cambridge, U.K. (2005), ISBN 0-85404-438–8.
[7] Compendium of Polymer Terminology and Nomenclature — IUPAC Recommendations 2008, R. G. Jones, J. Kahovec, R. Stepto, E. S. Wilks, M. Hess, T. Kitayama, W. V. Metanomski (Eds.), RSC Publishing, Cambridge, U.K. (2008), ISBN 978-0-85404-491–7.
[8] Section P-44 in ref. [5].
[9] R. S. Cahn, C. Ingold, V. Prelog. Angew. Chem. 78, 413 (1966); Angew. Chem., Int. Ed. Engl. 5, 385 and 511 (1966).
[10] V. Prelog, G. Helmchen. Angew. Chem. 94, 614 (1982); Angew. Chem., Int. Ed. Engl. 21, 567 (1982).
[11] ChemicalAbstractsService, https://www.cas.org.
[12] J. Brecher, K. N. Degtyarenko, H. Gottlieb, R. M. Hartshorn, K.-H. Hellwich, J. Kahovec, G. P. Moss, A. McNaught, J. Nyitrai, W. Powell, A. Smith, K. Taylor, W. Town, A. Williams, A. Yerin. Pure Appl. Chem. 80, 277 (2008).
[13] J. Brecher, K. N. Degtyarenko, H. Gottlieb, R. M. Hartshorn, G. P. Moss, P. Murray-Rust, J. Nyitrai, W. Powell, A. Smith, S. Stein, K. Taylor, W. Town, A. Williams, A. Yerin. Pure Appl. Chem. 78, 1897 (2006).
Return to other Brief Guides
IUPAC Chemical Nomenclature home page
Return to main IUBMB Biochemical Nomenclature home page