International Union of Pure and Applied Chemistry
Division VIII Chemical Nomenclature and Structure Representation Division
Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013.
Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4
Appendix 3. STRUCTURES FOR ALKALOIDS, STEROIDS, TERPENOIDS, AND SIMILAR COMPOUNDS
1. Alkaloids
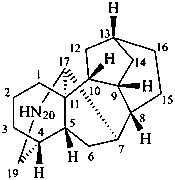
aconitane
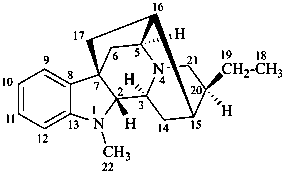
ajmalan
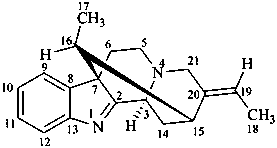
akuammilan
(named systematically by CAS)
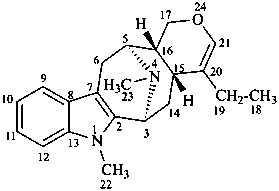
alstophyllan
(named systematically by CAS)
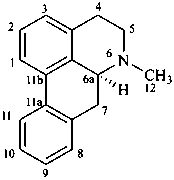
aporphine
(named systematically by CAS)
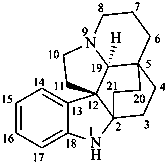
aspidofractinine
(named systematically by CAS)
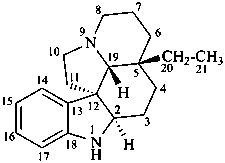
aspidospermidine
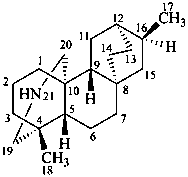
atidane
(named systematically by CAS)
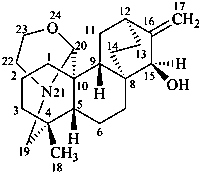
atisine
(named systematically by CAS)
berbaman
(named systematically by CAS)
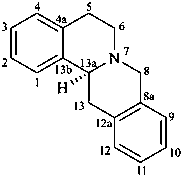
berbine
(named systematically by CAS)
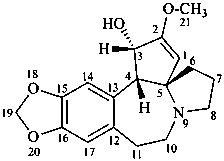
cephalotaxine
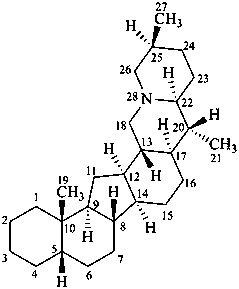
cevane
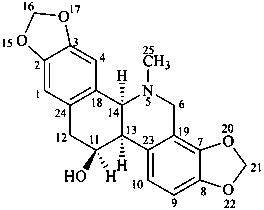
chelidonine
(named systematically by CAS)
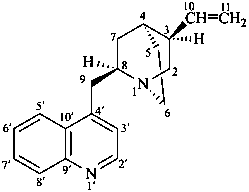
cinchonan
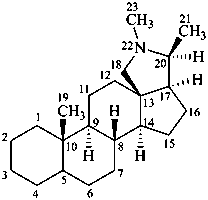
conanine
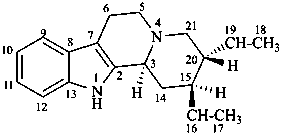
corynan
(named systematically by CAS)
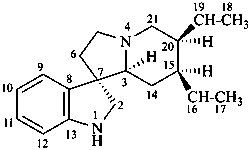
corynoxan
(named systematically by CAS)
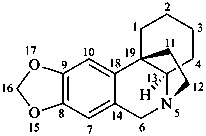
crinan
(named systematically by CAS)
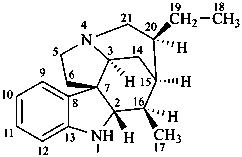
curan
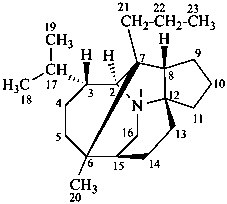
daphnane
(named systematically by CAS)
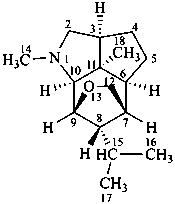
dendrobane
(named systematically by CAS)
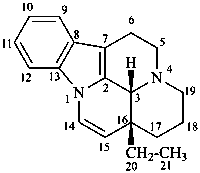
eburnamenine
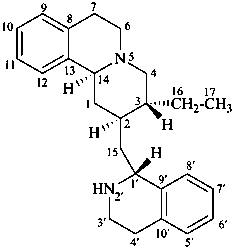
emetan
(named systematically by CAS)
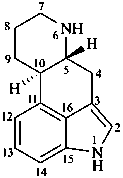
ergoline
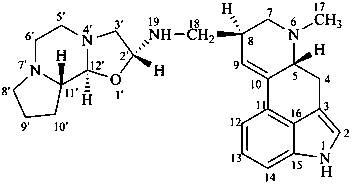
ergotaman
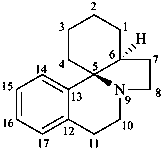
erythrinan
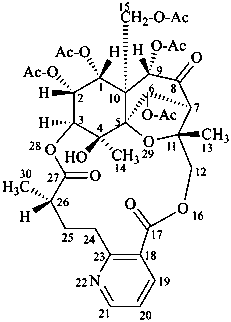
evonimine
(named systematically by CAS)
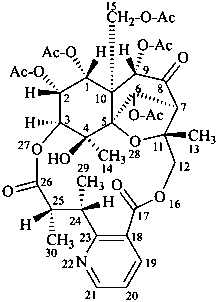
evonine
(named systematically by CAS)
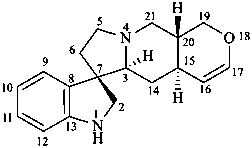
formosanan
(named systematically by CAS)
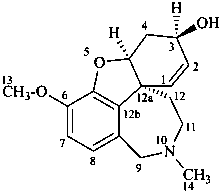
galanthamine
(named systematically by CAS)
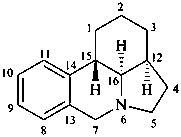
galanthan
(named systematically by CAS)
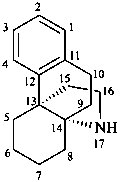
hasubanan
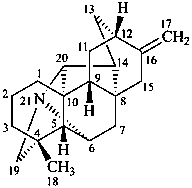
hetisan
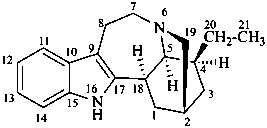
ibogamine
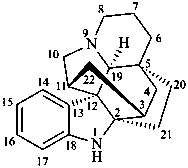
kopsan
(named systematically by CAS)
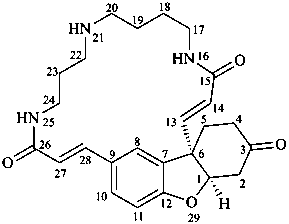
lunarine
(named systematically by CAS)
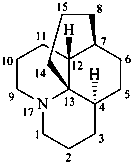
lycopodane
(named systematically by CAS)
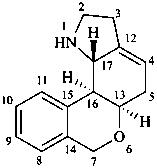
lycorenan
(named systematically by CAS)
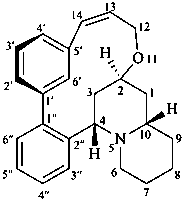
lythran
(named systematically by CAS)
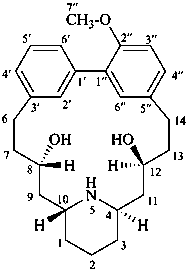
lythranidine
(named systematically by CAS)
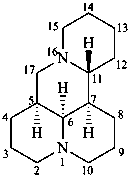
matridine
(named systematically by CAS)
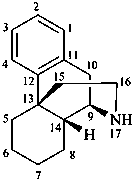
morphinan
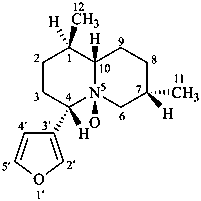
nupharidine
(named systematically by CAS)
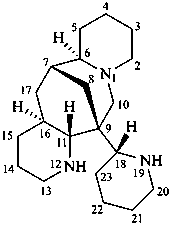
ormosanine
(named systematically by CAS)
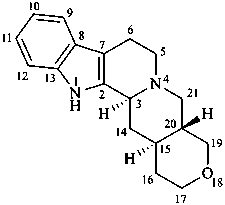
18-oxayohimban
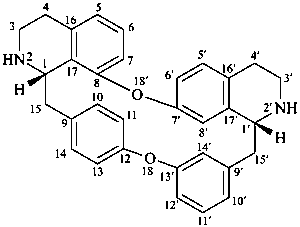
oxyacanthan
(named systematically by CAS)
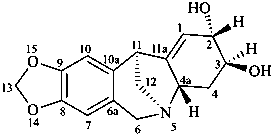
pancracine
(named systematically by CAS)
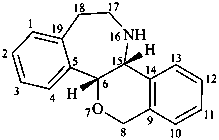
rheadan
(named systematically by CAS)
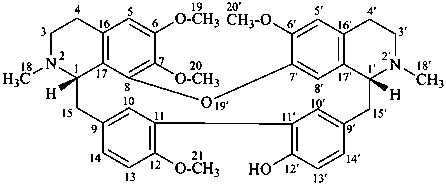
rodiasine
(named systematically by CAS)
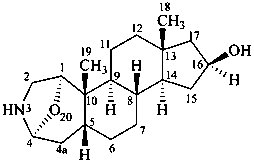
samandarine
(named systematically by CAS)
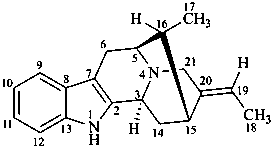
sarpagan
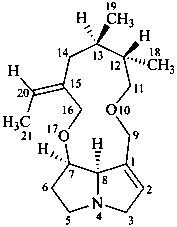
senecionan
(named systematically by CAS)
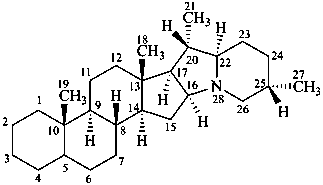
solanidane
(the CAS name requires the chirality at C-22 to be specified)
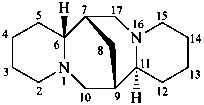
sparteine
(named systematically by CAS)
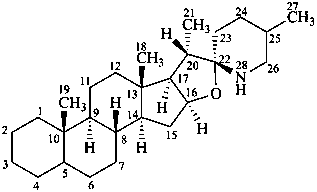
spirosolane
(the CAS name requires the chirality at C-22 to be specified)
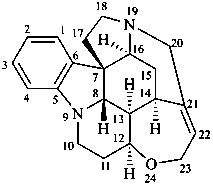
strychnidine
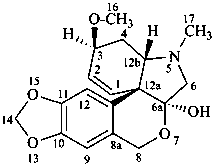
tazettine
(named systematically by CAS)
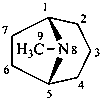
tropane
(named systematically by CAS)
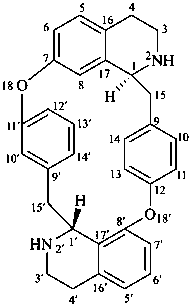
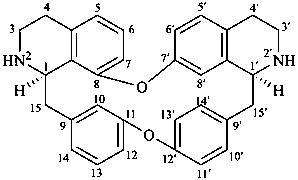
tubocuraran
(named systematically by CAS)
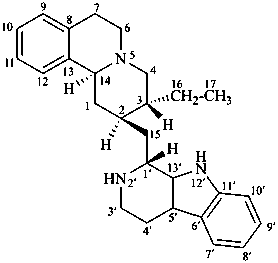
tubulosan
(named systematically by CAS)
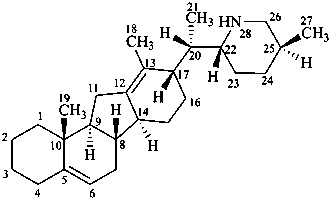
veratraman
(named systematically by CAS)
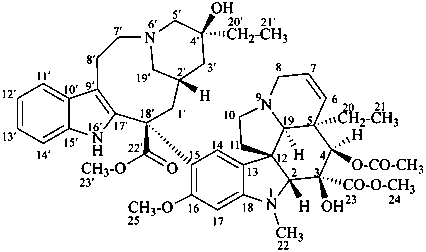
vincaleukoblastine
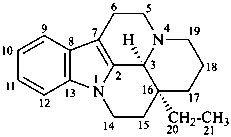
vincane
(CAS name based on eburnamenine)
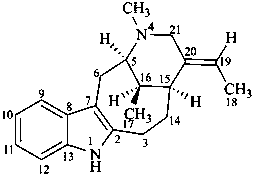
vobasan
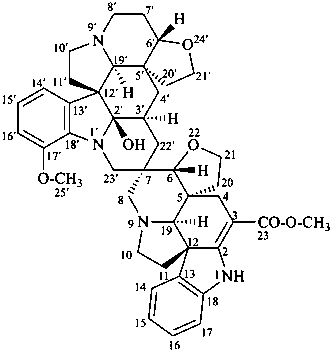
vobtusine
(named systematically by CAS)
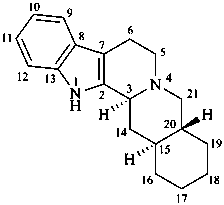
yohimban
2. Steroids
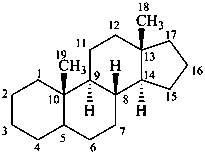
androstane
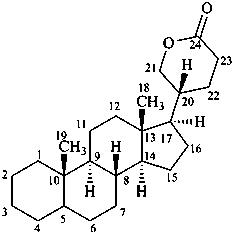
bufanolide
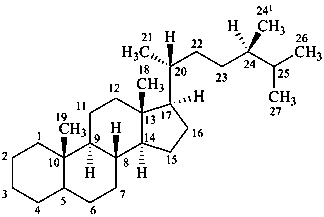
campestane
(named by CAS as a stereoisomer of ergostane in which the locant 241 is 28)
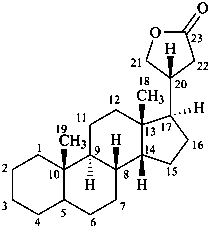
cardanolide
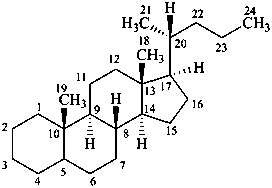
cholane
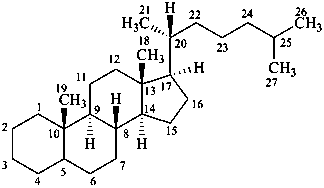
cholestane
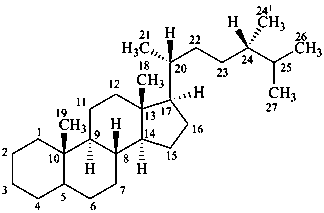
ergostane
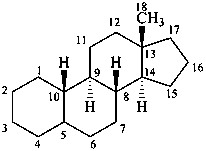
estrane
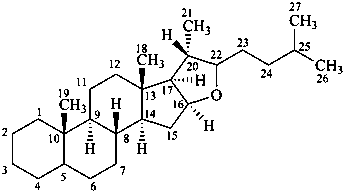
furostan
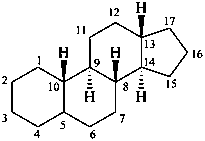
gonane
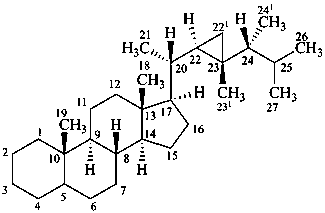
gorgostane
(for CAS the locants 221, 231, and 241 are 34, 33, and 28, respectively)
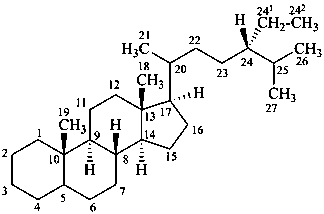
poriferastane
(named by CAS as a stereoisomer of stigmastane in which the locants 241 and 242 are 28 and 29, respectively.)
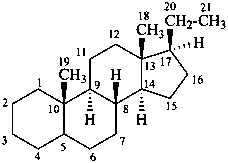
pregnane
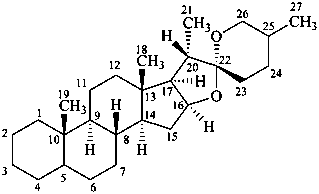
spirostan
(the CAS name requires the chirality at C-22 to be specified)
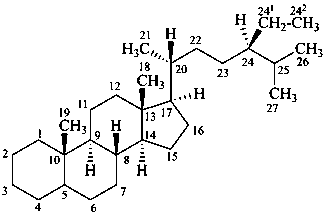
stigmastane
3. Terpenoids
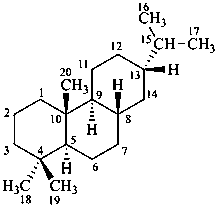
abietane
(named systematically by CAS)
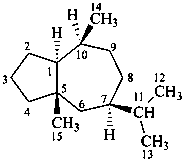
ambrosane
(named systematically by CAS)
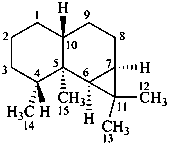
aristolane
(named systematically by CAS)
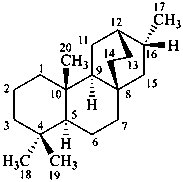
atisane
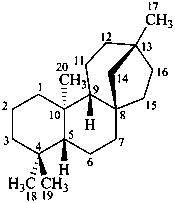
beyerane
(CAS name based on kaurane)
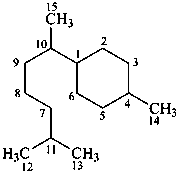
bisabolane
(named systematically by CAS)
bornane
(named systematically by CAS)
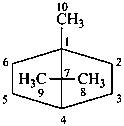
cadinane
(named systematically by CAS)
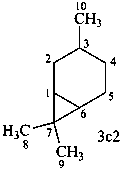
carane
(named systematically by CAS)
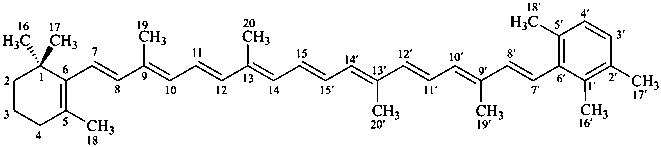
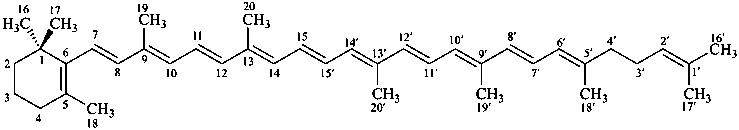
β,φ-carotene
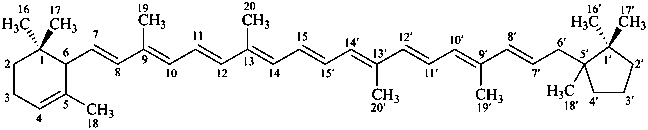
β,ψ-carotene
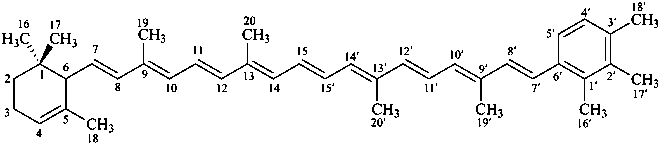
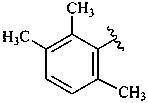
ε,κ-carotene
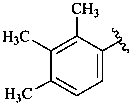
ε,χ-carotene
Note: There are 28 possible carotene parent structures of which four are illustrated above.
The 28 are derived from all permutations of the following seven end groups:
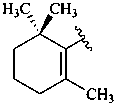 | 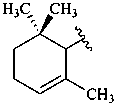 | 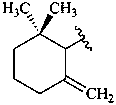 |  |
| β (beta) | ε (epsilon) | γ (gamma) | φ (phi) |
 | 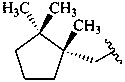 |  | |
| χ (chi) | κ (kappa) | ψ (psi) |
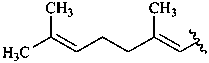
caryophyllane
(named systematically by CAS)
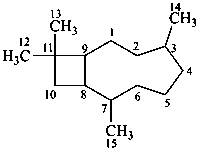
cedrane
(named systematically by CAS)
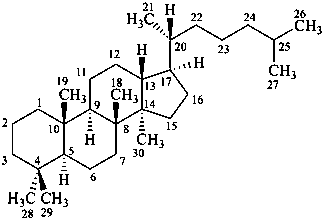
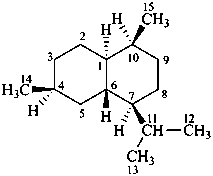
dammarane
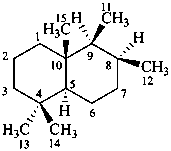
drimane
(named systematically by CAS)
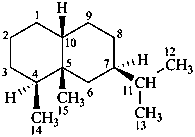
eremophilane
(named systematically by CAS)
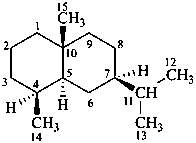
eudesmane
(named systematically by CAS)
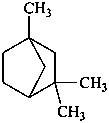
fenchane
(named systematically by CAS)
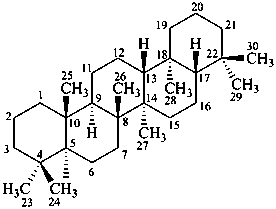
gammacerane
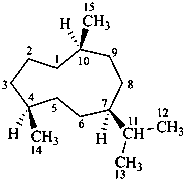
germacrane
(named systematically by CAS)
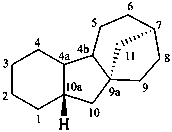
gibbane
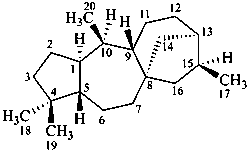
grayanotoxane
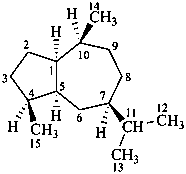
guaiane
(named systematically by CAS)
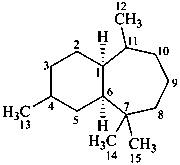
himachalane
(named systematically by CAS)
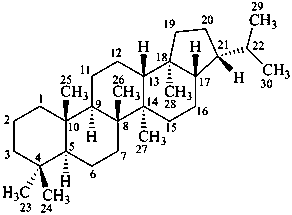
hopane
(CAS name based on gammacerane)
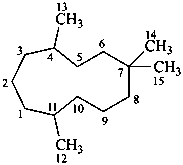
humulane
(named systematically by CAS)
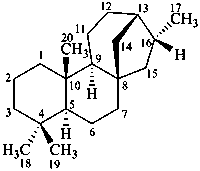
kaurane
(named by CAS ent-stereoisomer)
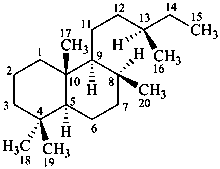
labdane
(named systematically by CAS)
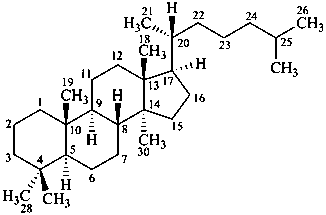
lanostane
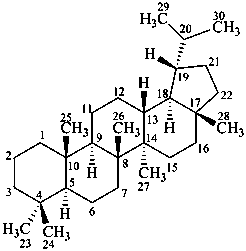
lupane
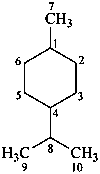
p-menthane
(named systematically by CAS)
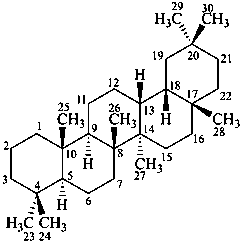
oleanane
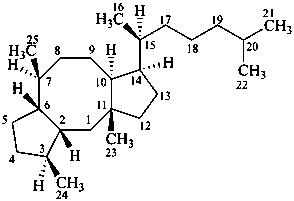
ophiobolane
(named systematically by CAS)
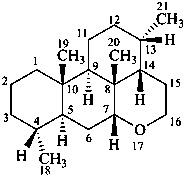
picrasane
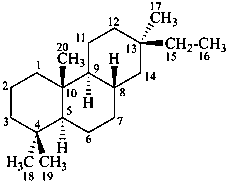
pimarane
(named systematically by CAS)
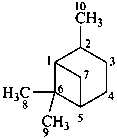
pinane
(named systematically by CAS)
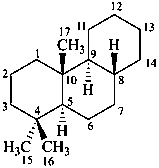
podocarpane
(named systematically by CAS)
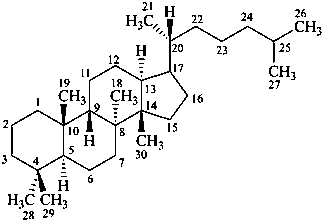
protostane
(named by CAS as a stereoisomer of dammarane)
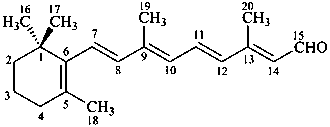
retinal
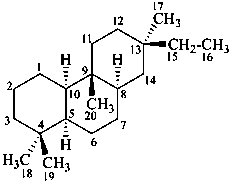
rosane
(named systematically by CAS)
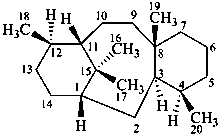
taxane
(named systematically by CAS)
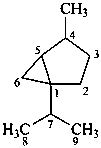
thujane
(named systematically by CAS)
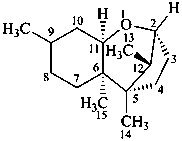
trichothecane
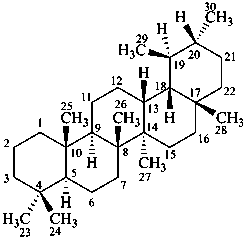
ursane
4..Miscellaneous
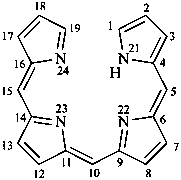
21H-biline
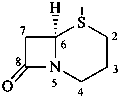
cepham
(named systematically by CAS)

corrin
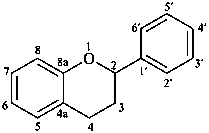
flavan
(named systematically by CAS)
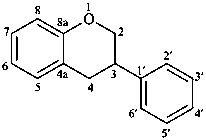
isoflavan
(named systematically by CAS)
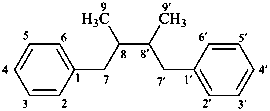
lignane
[only through a 8,8′ (β,β′) linkage]
(named systematically by CAS)
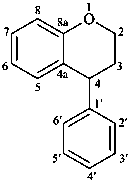
neoflavan
(named systematically by CAS)
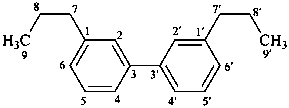
3,3′-neolignane
[and other structures not connected through a 8,8′) (β,β′) linkage]
(named systematically by CAS)
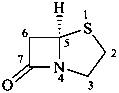
penam
(named systematically by CAS)
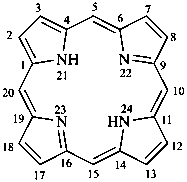
21H,23H-porphyrin
(named by CAS on the basis of the parent name porphine)
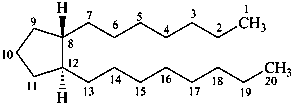
prostane
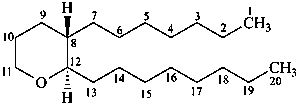
thromboxane
(named systematically by CAS)
Return to:
IUPAC Chemical Nomenclature home page.
Blue Book home page.


















































































































































