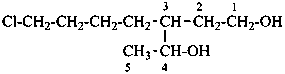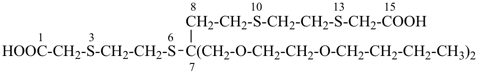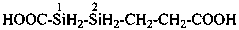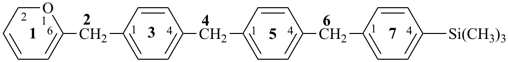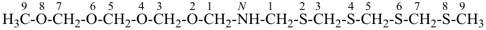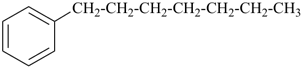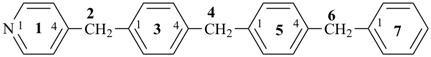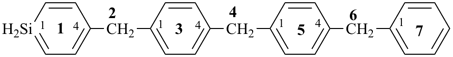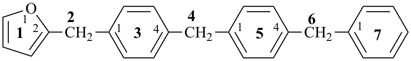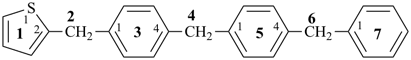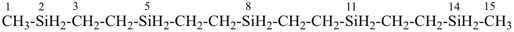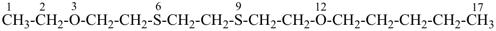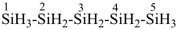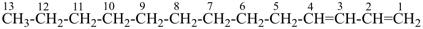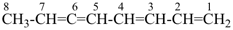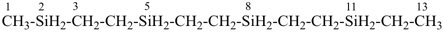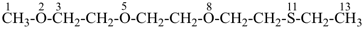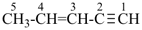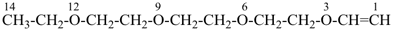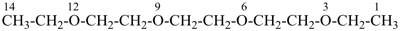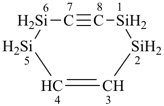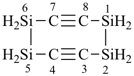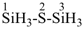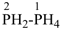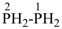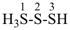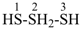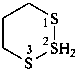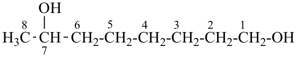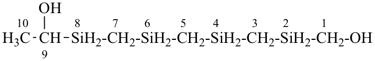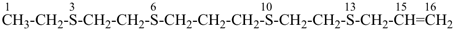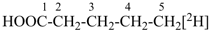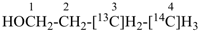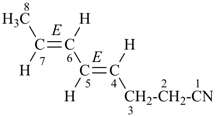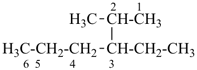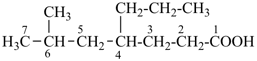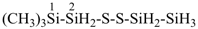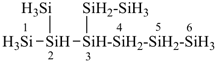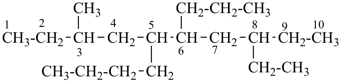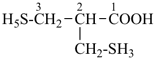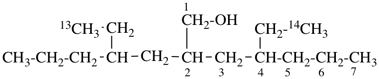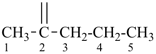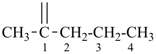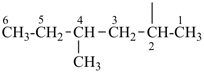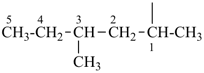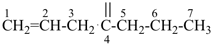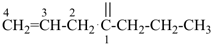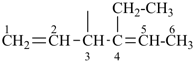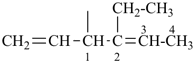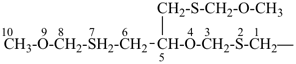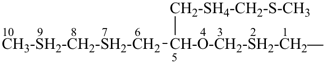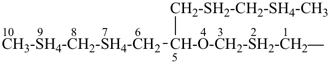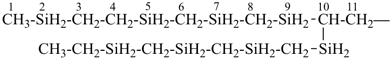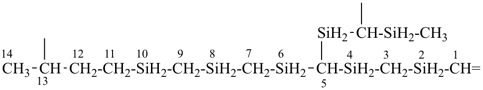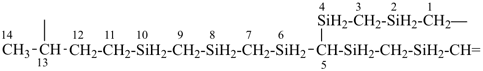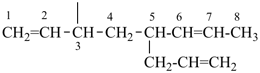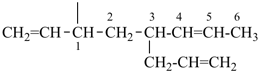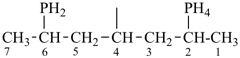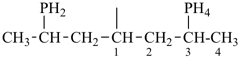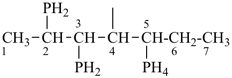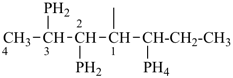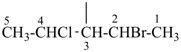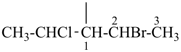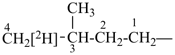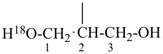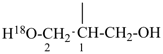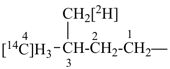International Union of Pure and Applied Chemistry
Division VIII Chemical Nomenclature and Structure Representation Division
Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013.
Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4
Chapter P-4 RULES FOR NAME CONSTRUCTION
P-40 Introduction
P-41 Seniority order for classes
P-42 Seniority order for acids
P-43 Seniority order for suffixes
P-44 Seniority order for parent structures
P-45 Selection of the preferred IUPAC name
P-46 The principal chain in substituent groups
P-40 INTRODUCTION
In this Chapter, principles regarding name construction are presented. It is recognized that in chemical discussions it may sometimes be convenient to depart from rigorous rules in order to provide a name more appropriate to the chemical intent or to avoid obscuring an important feature. However, such deviations should not be done without good reason, and names so derived are not recommended for general use in publications.
The application of the principles and rules described in this Chapter has been devised to lead to a preferred IUPAC name. Preferred IUPAC names are not necessarily the aim of a practicing chemist who wants to communicate with his colleagues in familiar, well understood terms, but it may be appropriate to those who cite chemical names in legislative documents, in international trade and commerce, or for preparing programs for index purposes, databases, and retrieval systems.
This Chapter includes the general rules and orders of seniority used in substitutive nomenclature and in other types of nomenclature when required.
P-41 SENIORITY ORDER FOR CLASSES
The order of seniority of classes is given in Table 4.1. It ranks classes expressed by suffixes (classes 1 through 20) and classes based on the senior atom in compounds (classes 21 through 43).
Table 4.1 General compound classes listed in decreasing order of seniority
1 Radicals
2 Radical anions
3 Radical cations
4 Anions
5 Zwitterions
6 Cations
7 Acids
7a ‘Suffix’ acids in the order carboxylic (not including carbonic or polycarbonic acids, which belong to sub-class ‘7b’), sulfonic, sulfinic, selenonic, seleninic, telluronic, tellurinic, each followed in turn by the corresponding peroxy, imidic, and hydrazonic acids. Chalcogen analogues follow each of the corresponding oxygen acids and, in each case, the chalcogen analogue with the greater number of the preferred chalcogen atom (O > S > Se > Te), considered first in −OOH groups, then in −OH groups as necessary. (See P-42 for the complete list of seniority of acids, P-43 for suffixes modified by functional replacement, and Table 4.4 for an extensive list of the order of seniority of all these suffixes.)
7b Carbon acids with no substitutable hydrogen atoms in the order polynuclear carbonic acids (tricarbonic acid, dicarbonic acid), then carbonic acid, and cyanic acid.
7c Oxoacids having substitutable hydrogen atoms attached to their central atoms and their acidic derivatives in the following decreasing order of seniority: azonic, azinic, phosphonic, phosphinic, phosphonous, phosphinous acids, etc. (see P-42 for the complete list).
7d Mononuclear and polynuclear oxoacids other than carbon acids without substitutable hydrogen atoms attached to their central atom (see 7b, above), but which may be functionalized or may form derivatives by functional replacement, which have substitutable hydrogen atoms.
7e Other monobasic ‘oxoacids’ used as functional parent compounds.
8 Anhydrides [substitutive nomenclature is used for cyclic anhydrides that are named as heterocycles (see 16 below); functional class names are given to acyclic anhydrides and to a few cyclic anhydrides corresponding to acids having retained names; cyclic anhydrides are preferred over noncyclic anhydrides, when functional names are used].
9 Esters (functional class names are given to noncyclic esters; lactones and other cyclic esters are named as heterocycles; see 16 below).
10 Acid halides and pseudohalides [first in the order of the corresponding acid given above, then in the order of the halogen atoms (–F > –Cl > –Br > –I); then in the following order of pseudohalogen groups ( –N3 > –CN > –NC > –NCO > –NCS > –NCSe > –NCTe > –CNO).
11 Amides [in the order of the corresponding acids; cyclic amides are named as heterocycles (see class 16 below)].
12 Hydrazides (in the order of the corresponding acids).
13 Imides (includes only cyclic imides derived from organic di- or polybasic acids having retained names).
14 Nitriles.
15 Aldehydes and chalcogen analogues.
16 Ketones (of the type –C-CO-C–), pseudoketones (of the type –C-CO-X , X-CO-X, or –CO-X-CO–, where X ≠ C, halogen, pseudohalogen, or NH2, see P-64.1.2) and heterones (see P-64.4). See Classes 8, 9, 11 and 13, on lactones, lactams, anhydrides, imides.
17 Hydroxy compounds and chalcogen analogues (includes alcohols and phenols, which no longer have a separate ranking order).
18 Hydroperoxides (peroxols), i.e., –OOH.
19 Amines (defined as having three single covalent attachments to a nitrogen atom, i.e., NR3).
20 Imines, R=NH or R=N-R′.
Classes denoted by the senior atom in heterane nomenclature
21 Nitrogen compounds: heterocycles, polyazanes, hydrazines (except for hydrazides), diazenes, hydroxylamines, azanes (except for amides, amines, and imines)
22 Phosphorus compounds: heterocycles, polyphosphanes, phosphanes
23 Arsenic compounds: heterocycles, polyarsanes, arsanes
24 Antimony compounds: heterocycles, polystibanes, stibanes
25 Bismuth compounds: heterocycles, polybismuthanes, bismuthanes
26 Silicon compounds: heterocycles, polysilanes, silanes
27 Germanium compounds: heterocycles, polygermanes, germanes
28 Tin compounds: heterocycles, polystannanes, stannanes
29 Lead compounds: heterocycles, polyplumbanes, plumbanes
30 Boron compounds: heterocycles, polyboranes, boranes
31 Aluminium compounds: heterocycles, polyalumanes, alumanes
32 Gallium compounds: heterocycles, polygallanes, gallanes
33 Indium compounds: heterocycles, polyindiganes, indiganes
34 Thallium compounds: heterocycles, polythallanes, thallanes
35 Oxygen compounds: heterocycles, polyoxidanes (trioxidane but not peroxides or ethers)
36 Sulfur compounds: heterocycles, polysulfanes (trisulfane, λ6 and λ4 mono and disulfanes, but not disulfides or sulfides)
37 Selenium compounds: heterocycles, polyselanes (triselane but not diselenides, selenides)
38 Tellurium compounds: heterocycles, polytellanes (tritellane but not ditellurides, tellurides)
39 λ7, λ5 and λ3 halogen compounds in the order F > Cl > Br > I.
40 Carbon compounds: rings, chains
41 Ethers, then sulfides, sulfoxides, sulfones; then selenides, selenoxides, etc.
42 Peroxides, then chalcogen analogues with the greater number of the senior chalcogen atom, where O > S > Se > Te.
43 λ1 Halogen compounds in the order F > Cl > Br > I
* In this table, the symbol ‘>’ means ‘is senior to’.
Examples:
HOOC-CH2-CH2–
2-carboxyethyl (preferred prefix)
[free valence = radical > carboxylic acid]
(CH3)3N+-CH2-COO–
(N,N-dimethylmethanaminiumyl)acetate (PIN)
(anion > cation)
HO-CH2-CH2-CONH2
3-hydroxypropanamide (PIN)
(amide > alcohol)
OHC-CH2-CH2-CN
4-oxobutanenitrile (PIN)
(nitrile > aldehyde)
HSSS-SiH3
trisulfanylsilane
(preselected name; see P-12.2) (Si > S)
BH2-PH2
boranylphosphane
(preselected name; see P-12.2) (P > B)
(CH3)4Si
tetramethylsilane (PIN)
(Si > C)
(C6H5)3P
triphenylphosphane (PIN)
(P > C)
CH3-O-CH2-S-CH3
methoxy(methylsulfanyl)methane (PIN)
(C > ether and sulfide)
CH3-SeSe-CH2-S-CH3
(methyldiselanyl)(methylsulfanyl)methane (PIN)
(C > diselenide and sulfide)
CH3-S-CH2-CH2-SO-CH3
1-(methanesulfinyl)-2-(methylsulfanyl)ethane (PIN)
(C > sulfide and sulfoxide)
P-42 SENIORITY ORDER FOR ACIDS
Acids of class 7 (see Table 4.1) in the order of classes of compounds are further classified into subclasses. These correspond to acids expressed by suffixes and acids used as functional parents (see P-34). The following subsections complete the brief description given in Class 7; the acids are described in decreasing seniority order.
P-42.1 Class 7a. Acids expressed by suffixes (excludes carbonic and polycarbonic acids)
P-42.2 Class 7b. Carbon acids with no substitutable hydrogen atoms
P-42.3 Class 7c. Noncarbon acids having substitutable hydrogen atoms on the central atom
P-42.4 Class 7d. Noncarbon acids used to generate derivatives having substitutable hydrogen atoms
P-42.5 Class 7e. Other monobasic ‘oxo’ acids used as functional parents.
P-42.1 CLASS 7a. ACIDS EXPRESSED BY SUFFIXES (EXCLUDES CARBONIC AND POLYCARBONIC ACIDS)
| carboxylic acids | –COOH | -carboxylic acid |
| –(C)O-OH | -oic acid |
| sulfonic acids | –SO2-OH | -sulfonic acid |
| sulfinic acids | –SO-OH | -sulfinic acid |
| selenonic acids | –SeO2-OH | -selenonic acid |
| seleninic acids | –SeO-OH | -seleninic acid |
| telluronic acids | –TeO2-OH | -telluronic acid |
| tellurinic acids | –TeO-OH | -tellurinic acid |
P-42.2 CLASS 7b. CARBON ACIDS WITH NO SUBSTITUTABLE HYDROGEN ATOMS
polycarbonic acids
dicarbonic acid
carbonic acid
cyanic acid
P-42.3 CLASS 7c. NONCARBON ACIDS HAVING SUBSTITUTABLE HYDROGEN ATOMS ON THE CENTRAL ATOM
All these names are preselected names. In this class, criteria for seniority are, in descending order:
(a) the central atom first in the list N > P > As > Sb > B;
(b) maximum number of central atoms;
(c) homopolyacids (isopolyacids) (see ref. 12);
(d) having contiguous central atoms;
(e) maximum number of acidic (–OH) groups;
(f) highest oxidation number for the central atom
| For consistency in the names of polynuclear oxoacids, the numerical infix ‘di’ has been uniformly used in naming dinuclear ‘hypo’ acids, for example hypodiphosphorous acid, rather than hypophosphorous acid. |
|
| azonic acid | NH(O)(OH)2 |
| azinic acid | NH2(O)(OH) |
| polyphosphonic acids | (HO)PH(O)-O-[PH(O)-O-]nPH(O)(OH) |
| diphosphonic acid | (HO)PH(O)-O-PH(O)(OH) |
hypodiphosphonic acid
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)(O)HP-PH(O)(OH) |
| phosphonic acid | PH(O)(OH)2 |
| polyphosphonous acids | (HO)PH-O-[PH-O-]nPH(OH) |
| diphosphonous acid | (HO)PH-O-PH(OH) |
hypodiphosphonous acid
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)HP-PH(OH) |
| phosphonous acid | PH(OH)2 |
| phosphinic acid | PH2(O)(OH) |
| phosphinous acid | PH2(OH) |
polyarsonic acids > diarsonic acid > hypodiarsonic acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| arsonic acid | AsH(O)(OH)2 |
polyarsonous acids > diarsonous acid > hypodiarsonous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| arsonous acid | AsH(OH)2 |
| arsinic acid | AsH2(O)(OH) |
| arsinous acid | AsH2(OH) |
polystibonic acids > distibonic acid > hypodistibonic acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| stibonic acid | SbH(O)(OH)2 |
polystibonous acids > distibonous acid > hypodistibonous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| stibonous acid | SbH(OH)2 |
| stibinic acid | SbH2(O)(OH) |
| stibinous acid | SbH2(OH) |
| diboronic acid | (HO)BH-O-BH(OH) |
hypodiboronic acid
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)HB-BH(OH) |
| boronic acid | BH(OH)2 |
| borinic acid | BH2(OH) |
P-42.4 CLASS 7d. NONCARBON ACIDS USED TO GENERATE DERIVATIVES HAVING SUBSTITUTABLE HYDROGEN ATOMS
All these names are preselected names.
In this class, criteria for seniority are, in descending order:
(a) the central atom first in the list: P > As > Sb > Si > B > S > Se >Te;
(b) maximum number of central atoms;
(c) homopolyacids (isopolyacids) (see ref. 12);
(d) polyacids having contiguous central atoms;
(e) maximum number of acidic groups (-OH groups);
(f) highest oxidation number for the central atom.
| polyphosphoric acids | (HO)2P(O)-O-[PO(OH)-O-]nP(O)(OH)2 |
| polyphosphorous acids | (HO)2P-O-[P(OH)-O-]n-P(OH)2 |
| tetraphosphoric acid | (HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)-O-P(O)(OH)2 |
| triphosphoric acid | (HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2 |
| diphosphoric acid | (HO)2P(O)-O-P(O)(OH)2 |
| diphosphorous acid | (HO)2P-O-P(OH)2 |
hypodiphosphoric acid
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)2(O)P-P(O)(OH)2 |
hypodiphosphorous acid
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)2P-P(OH)2 |
| phosphoric acid | P(O)(OH)3 |
| phosphorous acid | P(OH)3 |
polyarsoric acids > polyarsorous acids > diarsoric acid > diarsorous acid > hypodiarsoric acid > hypodiarsorous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| arsoric acid | As(O)(OH)3 |
| arsorous acid | As(OH)3 |
polystiboric acids > polystiborous acid > distiboric acid > distiborous acid > hypodistiboric acid > hypodistiborous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| stiboric acid | Sb(O)(OH)3 |
| stiborous acid | Sb(OH)3 |
| silicic acid | Si(OH)4 |
| (formerly orthosilicic acid. See P-67.1.1.1, P-67.1.2.2 and Table IR-8.1, ref. 12.) |
| diboric acid | (HO)2B-O-B(OH)2 |
hypodiboric acid
(for the prefix ‘hypodi’ see P-67.2.1)
| (HO)2B-B(OH)2 |
| boric acid | B(OH)3 |
| polysulfuric acids | (HO)SO2-O-[SO2-O-]nSO2(OH) |
| polysulfurous acids | (HO)SO-O-[SO-O-]nSO(OH) |
| disulfuric acid | (HO)SO2-O-SO2(OH) |
disulfurous acid (ambiguous see P-67.3.2)  | (HO)S(O)-O-S(O)(OH) |
dithionic acid (hypodisulfuric acid)
(for the prefix ‘hypodi’ see P-67.2.1) | (HO)O2S-SO2(OH) |
| dithionous acid (hypodisulfurous acid) | (HO)(O)S-S(O)(OH) |
| sulfuric acid | S(O)2(OH)2 |
| sulfurous acid | S(O)(OH)2 |
polyselenic acids > polyselenous acids > diselenic acid > diselenous acid > hypodiselenic acid > hypodiselenous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| selenic acid | Se(O)2(OH)2 |
| selenous acid | Se(O)(OH)2 |
polytelluric acids > polytellurous acids > ditelluric acid > ditellurous acid > hypoditelluric acid > hypoditellurous acid
(for the prefix ‘hypodi’ see P-67.2.1) |
| telluric acid | Te(O)2(OH)2 |
| tellurous acid | Te(O)(OH)2 |
P-42.5 CLASS 7e. OTHER MONOBASIC ‘OXO’ ACIDS USED AS FUNCTIONAL PARENTS
All these names are preselected names. In this class, criteria for seniority are, in descending order:
(a) the central atom first in the list N > F > Cl > Br > I;
(b) highest oxidation number for the central atom.
| nitric acid | HO-NO2 |
| nitrous acid | HO-NO |
| perfluoric acid | F(O)3OH |
| fluoric acid | F(O)2OH |
| fluorous acid | F(O)OH |
| hypofluorous acid | FOH |
| perchloric acid | Cl(O)3OH |
| chloric acid | Cl(O)2OH |
| chlorous acid | Cl(O)OH |
| hypochlorous acid | ClOH |
| perbromic acid | Br(O)3OH |
| bromic acid | Br(O)2OH |
| bromous acid | Br(O)OH |
| hypobromous acid | BrOH |
| periodic acid | I(O)3OH |
| iodic acid | I(O)2OH |
| iodous acid | I(O)OH |
| hypoiodous acid | IOH |
P-43 SENIORITY ORDER FOR SUFFIXES
P-43.0 Introduction
P-43.1 General methodology of functional replacement
P-43.0 INTRODUCTION
Suffixes are modified as indicated in Table 4.3 for acids and in Table 4.4 for all substituent suffixes. The order of seniority for suffixes is described in this Section. It is based on the seniority of classes 7 through 20 given in Table 4.1 and includes suffixes modified by functional replacement.
P-43.1 GENERAL METHODOLOGY OF FUNCTIONAL REPLACEMENT
Suffixes are modified as indicated in Table 4.3 for acids and in Table 4.4 for acids and other classes. Prefixes and infixes are used as indicated in Table 4.2. Prefixes are used to modify suffixes such as ‘ol’, ‘al’, for example ‘thiol’ and ‘thial’. Infixes are recommended to modify the suffixes ‘carboxylic acid’, ‘sulfonic acid’, and related suffixes, for example ‘carboperoxoic acid’ and ‘sulfonothioic acid’.
Table 4.2 Prefixes and infixes, in decreasing order of seniority, used to generate suffixes in preferred IUPAC names by functional replacement
| Prefix | Infix | Replaced atom(s) | Replacing atom(s) |
| peroxy- | -peroxo- | –O– | –OO– |
| thioperoxy- | -(thioperoxo)- | –O– | –OS– or –SO– |
| dithioperoxy- | -(dithioperoxo)- | –O– | –SS– |
| thio- | -thio- | –O– or =O | –S– or =S |
| seleno- | -seleno- | –O– or =O | –Se– or =Se |
| telluro- | -telluro- | –O– or =O | –Te– or =Te |
| imido- | -imido- | =O | =NH |
| hydrazono- | -hydrazono- | =O | =NNH2 |
When several oxygen atoms are replaceable, the following criteria are applied, in order, until a decision is reached:
(a) maximum number of oxygen atoms, then S, Se, and Te atoms, =NH and =NNH2 groups;
(b) maximum number of oxygen atoms, then S, Se, and Te atoms, in −OO− groups;
(c) oxygen atoms, then S, Se, and Te atoms, in −(O)OH and −OH groups.
The order of seniority is described, in the case of carboxylic acids, sulfonic acids and sulfinic acids, by indicating, after the name of the modified suffix the number and kind of atoms used in the replacement operation (see Table 4.3).
Table 4.3 gives the list, in decreasing order, of the seniority of suffixes and suffixes modified by functional replacement for carboxylic and sulfonic acids. Sulfinic acid suffixes are similar to sulfonic acids. Selenium and tellurium acid suffixes are formed by replacing ‘sulf’ by ‘selen’ or ‘tellur’.
Table 4.3 Carboxylic and sulfonic acid suffixes generated for IUPAC preferred names by functional replacement, in decreasing order of seniority
| 1. Carboxylic acids |
| –COOH | carboxylic acid | |
| –CO-OOH | carboperoxoic acid | (3O) |
| –CS-OOH | carboperoxothioic OO-acid | (2O,1S; OO) |
| –CSe-OOH | carboperoxoselenoic acid | (2O,1Se; OO) |
| –CO-SOH | carbo(thioperoxoic) SO-acid | (2O,1S; OS; OH) |
| –CO-OSH | carbo(thioperoxoic) OS-acid | (2O,1S; OS; SH) |
| –CO-SeOH | carbo(selenoperoxoic) SeO-acid | (2O,1Se; OSe; OH) |
| –CO-OSeH | carbo(selenoperoxoic) OSe-acid | (2O,1Se; OSe; SeH) |
| –CS-SOH | carbothio(thioperoxoic) SO-acid | (1O,2S; OS; OH) |
| –CS-OSH | carbothio(thioperoxoic) OS-acid | (1O,2S; OS; SH) |
| –CSe-OSH | carboseleno(thioperoxoic) OS-acid | (1O,1S,1Se;OS; SH) |
| –CS-SeOH | carbo(selenoperoxo)thioic SeO-acid | (1O,1S,1Se;OSe: OH) |
| –CS-OSeH | carbo(selenoperoxo)thioic OSe-acid | (1O,1S,1Se; OSe; SeH) |
| –CS-SSH | carbo(dithioperoxo)thioic acid | (3S) |
| –CSe-SeSeH | carbo(diselenoperoxo)selenoic acid | (3Se) |
| –CTe-TeTeH | carbo(ditelluroperoxo)telluroic acid | (3Te) |
| –CS-OH | carbothioic O-acid | (1O,1S; OH) |
| –CO-SH | carbothioic S-acid | (1O,1S; SH) |
| –CS-SH | carbodithioic acid | (2S) |
| –CSe-SH | carboselenothioic S-acid | (1S,1Se; SH) |
| –CS-SeH | carboselenothioic Se-acid | (1S,1Se; SeH) |
| –CSe-SeH | carbodiselenoic acid | (2Se) |
| –CTe-SeH | carboselenotelluroic Se-acid | (1Se,1Te; SeH) |
| –CTe-TeH | carboditelluroic acid | (2Te) |
| –C(=NH)-OH | carboximidic acid | |
| –C(=NH)-OOH | carboximidoperoxoic acid | (2O,1N; OO) |
| –C(=NH)-SOH | carboximido(thioperoxoic) SO-acid | (1O,1S,1N; OS; OH) |
| –C(=NH)-OSH | carboximido(thioperoxoic) OS-acid | (1O,1S,1N; OS; SH) |
| –C(=NH)-SSH | carbo(dithioperoxo)imidic acid | (2S,1N; SS) |
| –C(=NH)-SeSH | carboximido(selenothioperoxoic) SeS-acid | (1S,1Se,1N; SSe; SH) |
| –C(=NH)-SH | carboximidothioic acid | (1S,1N) |
| –C(=NH)-SeH | carboximidoselenoic acid | (1Se,1N) |
| –C(=NH)-TeH | carboximidotelluroic acid | (1Te,1N) |
| –C(=NNH2)-OH | carbohydrazonic acid | |
| –C(=NNH2)-OOH | carbohydrazonoperoxoic acid | (2O,1NN; OO) |
| –C(=NNH2)-SOH | carbohydrazono(thioperoxoic) SO-acid | (1O,1S,1NN; OS; OH) |
| –C(=NNH2)-OSH | carbohydrazono(thioperoxoic) OS-acid | (1O,1S,1NN; OS; SH) |
| –C(=NNH2)-TeTeH | carbo(ditelluroperoxo)hydrazonic acid | (2Te,1NN; TeTe) |
| 2. Sulfonic acids |
| –SO2-OH | sulfonic acid | |
| –SO2-OOH | sulfonoperoxoic acid | (4O) |
| –S(O)(S)-OOH | sulfonoperoxothioic OO-acid | (3O,1S; OO) |
| –S(O)(Se)-OOH | sulfonoperoxoselenoic OO-acid | (3O,1Se; OO) |
| –SO2-SOH | sulfono(thioperoxoic) SO-acid | (3O,1S; OS; OH) |
| –SO2-OSH | sulfono(thioperoxoic) OS-acid | (3O,1S; OS; SH) |
| –SS2-OOH | sulfonoperoxodithioic OO-acid | (2O,2S; OO) |
| –S(O)(S)-SOH | sulfonothio(thioperoxoic) SO-acid | (2O,2S; OS; OH) |
| –S(S)(Se)-OOH | sulfonoperoxoselenothioic OO-acid | (2O,1S,1Se; OO) |
| –SSeSe-SSH | sulfono(dithioperoxo)diselenoic acid | (2S,2Se; SS) |
| –SS2-SeSeH | sulfono(diselenoperoxo)dithioic acid | (2S,2Se; SeSe) |
| –STe2-TeTeH | sulfono(ditelluroperoxo)ditelluroic acid | (4Te) |
| –S(O)(S)-OH | sulfonothioic O-acid | (2O,1S; OH) |
| –SO2-SH | sulfonothioic S-acid | (2O,1S; SH) |
| –SO2-SeH | sulfonoselenoic Se-acid | (2O,1Se; SeH) |
| –SS2-OH | sulfonodithioic O-acid | (1O,2S; OH) |
| –S(O)(S)-SH | sulfonodithioic S-acid | (1O,2S; SH) |
| –S(Se)(Te)-OH | sulfonoselenotelluroic O-acid | (1O,1Se,1Te; OH) |
| –S(O)(Te)-SeH | sulfonoselenotelluroic Se-acid | (1O,1Se,1Te; SeH) |
| –S(O)(Se)-TeH | sulfonoselenotelluroic Te-acid | (1O,1Se.1Te; TeH) |
| –S(S2)-SH | sulfonotrithioic acid | (3S) |
| –S(O)(=NH)-OH | sulfonimidic acid | |
| –S(O)(=NH)-OOH | sulfonimidoperoxoic acid | (3O,1N; OO) |
| –S(S)(=NH)-OOH | sulfonimidoperoxothioic OO-acid | (2O,1S,1N; OO; OH) |
| –S(O)(=NH)-SOH | sulfonimido(thioperoxoic) SO-acid | (2O,1S,1N; OS; OH) |
| –S(O)(=NH)-OSH | sulfonimido(thioperoxoic) OS-acid | (2O,1S,1N; OS; SH) |
| –S(S)(=NH)-OH | sulfonimidothioic O-acid | (1O,1S; OH) |
| –S(O)(=NH)-SH | sulfonimidothioic S-acid | (1O,1S; SH) |
| –S(S)(=NH)-SH | sulfonimidodithioic acid | (2S) |
| –S(Se)(=NH)-SH | sulfonimidoselenothioic S-acid | (1S,1Se; SH) |
| –S(S)(=NH)-SeH | sulfonimidoselenothioic Se-acid | (1S,1Se; SeH) |
| –S(Te)(=NH)-TeH | sulfonimidoditelluroic acid | (2Te) |
| –S(=NH)2-OH | sulfonodiimidic acid | |
| –S(=NH)2-OOH | sulfonodiimidoperoxoic acid | (2O,2N; OO) |
| –S(=NH)2-SOH | sulfonodiimido(thioperoxoic) SO-acid | (1O,1S,2N: OS; OH) |
| –S(=NH)2-OSH | sulfonodiimido(thioperoxoic) OS-acid | (1O,1S,2N; OS; SH) |
| –S(=NH)2-SeH | sulfonodiimidoselenoic acid | (1Se,2N) |
| –S(=NH)2-TeH | sulfonodiimidotelluroic acid | (1Te,2N) |
| –S(O)(=NNH2)-OH | sulfonohydrazonic acid | |
| –S(O)(=NNH2)-OOH | sulfonohydrazonoperoxoic acid | (3O,1NN; OO) |
| –S(S)(=NNH2)-OOH | sulfonohydrazonoperoxothioic acid | (2O,1S,1NN; OO) |
| –S(S)(=NNH2)-OH | sulfonohydrazonothioic O-acid | (1O,1S,1NN; OH) |
| –S(O)(=NNH2)-SH | sulfonohydrazonothioic S-acid | (1O,1S,1NN; SH) |
| –S(=NNH2)2OH | sulfonodihyrazonic acid | |
| –S(=NNH2)2-OOH | sulfonodihydrazonoperoxoic acid | (2O,2NN; OO) |
| –S(=NNH2)2-SOH | sulfonodihydrazono(thioperoxoic) SO-acid | (1O,1S,2NN; SO, OH) |
| –S(=NNH2)2-SH | sulfonodihydrazonothioic acid | (1S,2NN) |
Table 4.4 Complete list of suffixes and functional replacement analogues (when present) for IUPAC preferred names, in decreasing order
of seniority
| 1. | Carboxylic acids | –COOH | carboxylic acid |
| | –(C)OOH | oic acid |
| |
| Carboperoxoic acids | –CO-OOH | carboperoxoic acid |
| | –(C)O-OOH | peroxoic acid |
| |
| Carboperoxoic acids modified by replacement with S, Se, and/or Te |
| | –CS-OOH | carboperoxothioic acid |
| | –(C)S-OOH | peroxothioic acid |
| |
| | –CSe-OOH | carboperoxoselenoic acid |
| | –(C)Se-OOH | peroxoselenoic acid |
| |
| | –CO-SOH | carbo(thioperoxoic) SO-acid |
| | –(C)O-SOH | (thioperoxoic) SO-acid |
| |
| | –CO-OSH | carbo(thioperoxoic) OS-acid |
| | –(C)O-OSH | (thioperoxoic) OS-acid |
| |
| Carboxylic acids modified by replacement with S, Se, and/or Te |
| | –CS-OH | carbothioic O-acid |
| | –(C)S-OH | thioic O-acid |
| |
| | –CO-SH | carbothioic S-acid |
| | –(C)O-SH | thioic S-acid |
| |
| | –CO-SeH | carboselenoic Se-acid |
| | –(C)O-SeH | selenoic Se-acid |
| |
| | –CS-SH | carbodithioic acid |
| | –(C)S-SH | dithioic acid |
| |
| 2. | Carboximidic acids | –C(=NH)-OH | carboximidic acid |
| | –(C)(=NH)-OH | imidic acid |
| |
| Carboximidoperoxoic acids | –C(=NH)-OOH | carboximidoperoxoic acid |
| | –(C)(=NH)-OOH | imidoperoxoic acid |
| |
| Carboximidoperoxoic acids modified by replacement with S, Se, and/or Te |
| | –C(=NH)-SOH | carboximido(thioperoxoic) SO-acid |
| | –(C)(=NH)-SOH | imido(thioperoxoic) SO-acid |
| |
| | –C(=NH)-OSH | carboximido(thioperoxoic) OS-acid |
| | –(C)(=NH)-OSH | imido(thioperoxoic) OS-acid |
| |
| | –C(=NH)-SSH | carbo(dithioperox)imidic acid |
| | –(C)(=NH)-SSH | (dithioperox)imidic acid |
| |
| | –C(=NH)-SeSH | carboximido(selenothioperoxoic) SeS-acid |
| | –(C)(=NH)-SeSH | imido(selenothioperoxoic) SeS-acid |
| |
| Carboximidic acids modified by replacement with S, Se, and/or Te |
| | –C(=NH)-SH | carboximidothioic acid |
| | –(C)(=NH)-SH | imidothioic acid |
| |
| 3. | Carbohydrazonic acids | –C(=NNH2)-OH | carbohydrazonic acid |
| | –(C)(=NNH2)-OH | hydrazonic acid |
| |
| Carbohydrazonoperoxoic acids | –C(=NNH2)-OOH | carbohydrazonoperoxoic acid |
| | –(C)(=NNH2)-OOH | hydrazonoperoxoic acid |
| |
| Carbohydrazonoperoxoic acids modified by replacement with S, Se, and/or Te |
| | –C(=NNH2)-SOH | carbohydrazono(thioperoxoic) SO-acid |
| | –(C)(=NNH2)-SOH | hydrazono(thioperoxoic) SO-acid |
| |
| | –C(=NNH2)-OSH | carbohydrazono(thioperoxoic) OS-acid |
| | –(C)(=NNH2)-OSH | hydrazono(thioperoxoic) OS-acid |
| |
| | –C(=NNH2)-TeTeH | carbo(ditelluroperoxo)hydrazonic acid |
| | –(C)(=NNH2)-TeTeH | (ditelluroperoxo)hydrazonic acid |
| |
| Carbohydrazonic acids modified by replacement with S, Se, and/or Te |
| | –C(=NNH2)-SH | carbohydrazonothioic acid |
| | –(C)(=NNH2)-SH | hydrazonothioic acid |
| |
| 4. | Sulfonic acids | –SO2-OH | sulfonic acid |
| Sulfonoperoxoic acids | –SO2-OOH | sulfonoperoxoic acid |
| |
| Sulfonoperoxoic acids modified by replacement with S, Se and/or Te |
| | –S(O)(S)-OOH | sulfonoperoxothioic acid |
| | –SO2-SOH | sulfono(thioperoxoic) SO-acid |
| | –SO2-OSH | sulfono(thioperoxoic) OS-acid |
| | –SS2-OOH | sulfonoperoxodithioic acid |
| |
| Sulfonic acids modified by replacement with S, Se and/or Te |
| | –SO2-SH | sulfonothioic S-acid |
| | –S(O)(S)-OH | sulfonothioic O-acid |
| | –S(S)(S)-SH | sulfonotrithioic acid |
| |
| 5. | Sulfonimidic acids | –S(O)(=NH)-OH | sulfonimidic acid |
| |
| Sulfonimidoperoxoic acids | –S(O)(=NH)-OOH | sulfonimidoperoxoic acid |
| |
| Sulfonimidoperoxoic acids modified by replacement with S, Se, or Te |
| | –S(O)(=NH)-SOH | sulfonimido(thioperoxoic) SO-acid |
| | –S(O)(=NH)-OSH | sulfonimido(thioperoxoic) OS-acid |
| |
| Sulfonimidic acids modified by replacement with S, Se or Te |
| | –S(O)(=NH)-SH | sulfonimidothioic S-acid |
| |
| 6. | Sulfonodiimidic acids | –S(=NH)2-OH | sulfonodiimidic acid |
| |
| Sulfonodiimidoperoxoic acids | –S(=NH)2-OOH | sulfonodiimidoperoxoic acid |
| |
| Sulfonodiimidoperoxoic acids modified by replacement with S, Se, and/or Te |
| | –S(=NH)2-SOH | sulfonodiimido(thioperoxoic) SO-acid |
| | –S(=NH)2-OSH | sulfonodiimido(thioperoxoic) OS-acid |
| |
| Sulfonodiimidic acids modified by replacement with S, Se, and/or Te |
| | –S(=NH)2-SeH | sulfonodiimidoselenoic acid |
| |
| 7. | Sulfonohydrazonic acids | –S(O)(=NNH2)-OH | sulfonohydrazonic acid |
| |
| Sulfonohydrazonoperoxoic acids | –S(O)(=NNH2)-OOH | sulfonohydrazonoperoxoic acid |
| |
| Sulfonohydrazonoperoxoic acids modified by replacement with S, Se, and/or Te |
| | –S(S)(=NNH2)-OOH | sulfonohydrazonoperoxothioic acid |
| |
| Sulfonohydrazonic acids modified by replacement with S, Se and/or Te |
| | –S(S)(=NNH2)-OH | sulfonohydrazonothioic O-acid |
| | –S(O)(=NNH2)-SH | sulfonohydrazonothioic S-acid |
| |
| 8. | Sulfonodihydrazonic acids | –S(=NNH2)2-OH | sulfonodihydrazonic acids |
| |
| Sulfonodihydrazonoperoxoic acid | –S(=NNH2)2-OOH | sulfonodihydrazonoperoxoic acid |
| |
| Sulfonodihydrazonoperoxoic acids modified by replacement with S, Se, and/or Te |
| | –S(=NNH2)2-SOH | sulfonodihydrazono(thioperoxoic) SO-acid |
| |
| Sulfonodihydrazonic acids modified by replacement with S, Se, and/or Te |
| | –S(=NNH2)2-SH | sulfonodihydrazonothioic acid |
| |
| 9. | Sulfinic acids | –SO-OH | sulfinic acid |
| |
| Sulfinoperoxoic acid | –SO-OOH | sulfinoperoxoic acid |
| |
| Sulfinoperoxoic acid modified by replacement with S, Se, and/or Te |
| | –S(S)-OOH | sulfinoperoxothioic acid |
| | –SO-SOH | sulfino(thioperoxoic) SO-acid |
| | –SO-OSH | sulfino(thioperoxoic) OS-acid |
| |
| Sulfinic acids modified by replacement with S, Se, and/or Te |
| | –SS-OH | sulfinothioic O-acid |
| | –SO-SeH | sulfinoselenoic Se-acid |
| |
| 10. | Sulfinimidic acids | –S(=NH)-OH | sulfinimidic acid |
| |
| Sulfinimidoperoxoic acids | –(=NH)-OOH | sulfinimidoperoxoic acid |
| |
| Sulfinimidoperoxoic acids modified by replacement with S, Se and/or Te |
| | –S(=NH)-OSH | sulfinimido(thioperoxoic) OS-acid |
| |
| Sulfinimidic acids modified by replacement with S, Se, and/or Te |
| | –S(=NH)-SH | sulfinimidothioic acid |
| |
| 11. | Sulfinohydrazonic acids | –S(=NNH2)-OH | sulfinohydrazonic acid |
| |
| Sulfinohydrazonoperoxoic acids | –S(=NNH2)-OOH | sulfinohydrazonoperoxoic acid |
| |
| Sulfinohydrazonoperoxoic acids modified by replacement with S, Se and/or Te |
| | –S(=NNH2)-SSeH | sulfinohydrazono(selenothioperoxoic) SSe-acid |
| |
| Sulfinohydrazonic acids modified by replacement with S, Se, and/or Te |
| | –S(=NNH2)-TeH | sulfinohydrazonotelluroic acid |
| |
| 12. | Selenonic acids | –SeO2-OH | selenonic acid (as for sulfonic acids) |
| |
| 13. | Seleninic acids | –SeO-OH | seleninic acid (as for sulfinic acids) |
| |
| 14. | Telluronic acids | –TeO2-OH | telluronic acid (as for sulfonic acids) |
| |
| 15. | Tellurinic acids | –TeO-OH | tellurinic acid (as for sulfinic acids) |
| |
| 16. | Carboxamides | –CO-NH2 | carboxamide |
| | –(C)O-NH2 | amide |
| |
| Carboxamides modified by replacement with S, Se, and/or Te |
| | –CS-NH2 | carbothioamide |
| | –(C)S-NH2 | thioamide |
| |
| 17. | Carboximidamides | –C(=NH)-NH2 | carboximidamide |
| | –(C)(=NH)-NH2 | imidamide |
| |
| 18. | Carbohydrazonamides | –C(=NNH2)-NH2 | carbohydrazonamide |
| | –(C)(=NNH2)-NH2 | hydrazonamide |
| |
| 19. | Sulfonamides | –SO2-NH2 | sulfonamide |
| |
| Sulfonamides modified by replacement with S, Se, and/or Te |
| | –S(O)(S)-NH2 | sulfonothioamide |
| | –S(S)(Se)-NH2 | sulfonoselenothioamide |
| |
| 20. | Sulfonimidamides | –S(O)(=NH)-NH2 | sulfonimidamide |
| |
| Sulfonimidamides modified by replacement with S, Se, and/or Te |
| | –S(S)(=NH)-NH2 | sulfonimidothioamide |
| |
| 21. | Sulfonodiimidamides | –S(=NH)2-NH2 | sulfonodiimidamide |
| |
| 22. | Sulfonohydrazonamides | –S(O)(=NNH2)-NH2 | sulfonohydrazonamide |
| |
| Sulfonohydrazonamides modified by replacement with S, Se, and/or Te |
| | –S(S)(=NNH2)-NH2 | sulfonohydrazonothioamide |
| |
| 23. | Sulfonodihydrazonamides | –S(=NNH2)2-NH2 | sulfonodihydrazonamide |
| |
| 24. | Sulfinamides | –SO-NH2 | sulfinamide |
| |
| Sulfinamides modified by replacement with S, Se, and/or Te |
| | –S(Se)-NH2 | sulfinoselenoamide |
| |
| 25. | Sulfinimidamides | –S(=NH)-NH2 | sulfinimidamide |
| |
| 26. | Sulfinohydrazonamides | –S(=NNH2)-NH2 | sulfinohydrazonamide |
| |
| 27. | Selenonamides | –SeO2-NH2 | selenonamide (as for sulfonamides) |
| |
| 28. | Seleninamides | –SeO-NH2 | seleninamide (as for sulfinamides) |
| |
| 29. | Telluronamides | –TeO2-NH2 | telluronamide (as for sulfonamides) |
| |
| 30. | Tellurinamides | –TeO-NH2 | tellurinamide (as for sulfinamide) |
| |
| 31. | Carbohydrazides | –CO-NHNH2 | carbohydrazide |
| | –(C)O-NHNH2 | hydrazide |
| |
| Carbohydrazides modified by replacement with S, Se, and/or Te |
| | –CS-NHNH2 | carbothiohydrazide |
| |
| 32. | Carboximidohydrazides | –C(=NH)-NHNH2 | carboximidohydrazide |
| | –(C)(=NH)-NHNH2 | imidohydrazide |
| |
| 33. | Carbohydrazonohydrazides | –C(=NNH2)-NHNH2 | carbohydrazonohydrazide |
| | –(C)(=NNH2)-NHNH2 | hydrazonohydrazide |
| |
| 34. | Sulfonohydrazides | –SO2-NHNH2 | sulfonohydrazide |
| |
| Sulfonohydrazides modified by replacement with S, Se, and/or Te |
| | –S(O)(S)-NHNH2 | sulfonothiohydrazide |
| |
| 35. | Sulfonimidohydrazides | –S(O)(=NH)-NHNH2 | sulfonimidohydrazide |
| |
| Sulfonimidohydrazides modified by replacement with S, Se, and/or Te |
| | –S(Se)(=NH)-NHNH2 | sulfonimidoselenohydrazide |
| |
| 36. | Sulfonodiimidohydrazides | –S(=NH)2-NHNH2 | sulfonodiimidohydrazide |
| |
| 37. | Sulfonohydrazonohydrazides | –S(O)(=NNH2)-NHNH2 | sulfonohydrazonohydrazide |
| |
| Sulfonohydrazonohydrazides modified by replacement with S, Se, andor Te |
| | –S(Te)(=NNH2)-NHNH2 | sulfonohydrazonotellurohydrazide |
| |
| 38. | Sulfonodihydrazonohydrazides | –S(=NNH2)2-NHNH2 | sulfonodihydrazonohydrazide |
| |
| 39. | Sulfinohydrazides | –S(O)-NHNH2 | sulfinohydrazide |
| |
| Sulfinohydrazides modified by replacement with S, Se, and/or Te |
| | –S(Se)-NHNH2 | sulfinoselenohydrazide |
| |
| 40. | Sulfinimidohydrazides | –S(=NH)-NHNH2 | sulfinimidohydrazide |
| |
| 41. | Sulfinohydrazonohydrazides | –S(=NNH2)-NHNH2 | sulfinohydrazonohydrazide |
| |
| 42. | Selenonohydrazides | –SeO2-NHNH2 | selenonohydrazide (as for sulfonohydrazides) |
| |
| 43. | Seleninohydrazides | –Se(O)-NHNH2 | seleninohydrazide (as for sulfinohydrazides) |
| |
| 44. | Telluronohydrazides | –TeO2-NHNH2 | telluronohydrazide (as for sulfonohydrazides) |
| |
| 45. | Tellurinohydrazides | –Te(O)-NHNH2 | tellurinohydrazide (as for sulfinohydrazides) |
| |
| 46. | Nitriles | –CN | carbonitrile |
| | –(C)N | nitrile |
| |
| 47. | Aldehydes | –CHO | carbaldehyde |
| | –(C)HO | al |
| |
| Aldehydes modified by replacement with S, Se, and/or Te |
| | –CHS | carbothialdehyde |
| | –(C)HS | thial |
| |
| | –CHSe | carboselenaldehyde |
| | –(C)HSe | selenal |
| |
| | –CHTe | carbotelluraldehyde |
| | –(C)HTe | tellural |
| |
| 48. | Ketones, pseudoketones, and heterones | >(C)=O | one |
| |
| Ketones, pseudoketones, and heterones modified by replacement with S, Se, and/or Te |
| | >(C)=S | thione |
| | >(C)=Se | selone (not selenone) |
| | >(C)=Te | tellone (not tellurone) |
| |
| 49. | Hydroxy compounds | –OH | ol |
| |
| Hydroxy compounds modified by replacement with S, Se, and/or Te |
| | –SH | thiol |
| | –SeH | selenol |
| | –TeH | tellurol |
| |
| 50. | Hydroperoxides | –OOH | peroxol |
| |
| Hydroperoxides modified by replacement with S, Se, and/or Te |
| | –OSH | OS-thioperoxol |
| | –SOH | SO-thioperoxol (not sulfenic acid) |
| |
| 51. | Amines | –NH2 | amine |
| |
| 52. | Imines | =NH | imine |
P-44 SENIORITY ORDER FOR PARENT STRUCTURES
A parent structure is defined (P-15.1.1 and P-15.1.2) as a parent hydride, for example methane, a functionalized parent hydride, for example, cyclohexanol, or a functional parent compound, for example, acetic acid. For choice of the preferred IUPAC name based on a senior parent structure, see P-45.
P-44.0 Introduction
P-44.1 Seniority order for parent structures
P-44.2 Seniority order only for rings and ring systems
P-44.3 Seniority of acyclic chains (the principal chain)
P-44.4 Seniority criteria applicable to rings, ring systems, or acyclic chains
P-44.0 INTRODUCTION
The selection of a preferred parent structure is based on the seniority of classes (see P-41), which gives priority first to characteristic groups expressed as suffixes and then to parent hydrides when different classes are present. Section P-44.1 covers the selection of a preferred parent structure when different classes are involved and the selection between rings and chains in the same class. When there is a choice among cyclic parent hydrides, the senior ring or ring system is chosen in accord with the seniority order of rings and ring systems (see P-44.2). When there is a choice among acyclic parent hydrides a principal chain must be chosen (see P-44.3). The three seniority orders, for classes, rings and ring systems, and the principal chain, are expressed in a general seniority order called ‘seniority order for parent structures’. Section P-44.4 covers criteria for selection of a senior parent structure applicable to either rings, ring systems, or acyclic chains.
A thorough revision and extension of the seniority order of classes, of rings and ring systems, and for selecting the principal chain was needed in the context of preferred names. This revision incorporates a major change from earlier recommendations given in the 1979 edition (ref. 1) and the 1993 Guide (ref. 2).
| In acyclic parent structures the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second criterion. |
|
Note 1: Since the senior parent structure can occur in a compound in multiple ways, several plausible, unambiguous names can be generated. The criteria necessary to select a preferred IUPAC name are described in P-45; thus, traditional criteria related to substituent groups are not incorporated in this Section.
Note 2: Criteria related to nonstandard bonding have been incorporated into the criteria applicable to either rings, ring systems, or acyclic chains (P-44.4); hierarchically, they come after the criteria related to unsaturation (double bonds) and before those related to indicated hydrogen atoms.
P-44.1 SENIORITY ORDER FOR PARENT STRUCTURES
When there is a choice, the senior parent structure is chosen by applying the following criteria, in order, until a decision is reached. These criteria must always be applied before those applicable to rings and ring systems (see P-44.2) and to chains (see P-44.3). Then criteria applicable to both chains and rings or ring systems given in P-44.4 are considered.
P-44.1.1 The senior parent structure has the maximum number of substituents corresponding to the principal characteristic group (suffix) or senior parent hydride in accord with the seniority of classes (P-41) and the seniority of suffixes (P-43).
Examples:
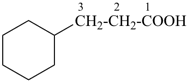
3-cyclohexylpropanoic acid (PIN)
cyclohexanepropanoic acid (see P-15.6)
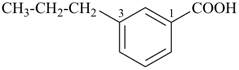
3-propylbenzoic acid (PIN)
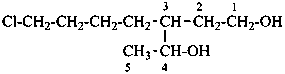
3-(4-chlorobutyl)pentane-1,4-diol (PIN)
[not 7-chloro-3-(1-hydroxyethyl)heptan-1-ol;
there are two of the principal characteristic groups
in the parent of the PIN and only one in the other name]
[not 3-(4-chlorobutyl)pentane-2,5-diol;
the locant set ‘1,4’ for the principal characteristic groups
of the PIN is lower than ‘2,5’]
NH2-NH-COOH
hydrazinecarboxylic acid (PIN)
H3Si-CH2-CH2-COOH
3-silylpropanoic acid (PIN)
HOOC-SiH2-CH2-CH3
ethylsilanecarboxylic acid (PIN)
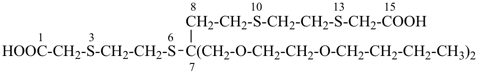
7,7-bis[(2-butoxyethoxy)methyl]-3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
[not 9-[(2-butoxyethoxy)methyl]-9-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)-11,14-dioxa-3,6-dithiaoctadecan-1-oic acid;
nor 7-[(2-butoxyethoxy)methyl]-7-[2-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)ethyl]-9,12-dioxa-3,6-dithiahexadecan-1-oic acid;
there are two of the principal characteristic group in the parent of the PIN and only one in the other names]
H3Si-CH2-CH2-SiH2-CH2-CH2-SiH3
[silanediyldi(ethane-2,1-diyl)]bis(silane) (PIN)
(Si is senior to C, see P-44.1.2)
[not bis(2-silylethyl)silane;
the multiplicative preferred name expresses two occurrences of the parent hydride silane;
the substitutive name expresses only one]
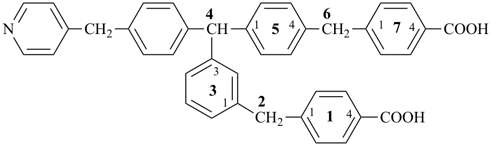
4-{4-[(pyridin-4-yl)methyl]phenyl}-1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane-14,74-dicarboxylic acid (PIN)
[not 4-{[4-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane-74-carboxylic acid;
nor 4-{[3-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane-74-carboxylic acid;
there are two of the principal characteristic group in the PIN and only one in the other names]
P-44.1.2 The senior parent structure, whether cyclic or acyclic, has the senior atom in accordance with the seniority of classes (see P-41) expressed by the following decreasing element order: N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl > O > S > Se > Te > C. This criterion is applied to select the senior atom in parents and to choose between rings and chains. It is not used to choose between rings or to select the principal chain modified by skeletal replacement (‘a’) nomenclature. If the most senior class is a ring system or a chain modified by skeletal replacement (‘a’) nomenclature, the senior parent is chosen among all rings (see P-44.2) or chains (see P-44.3) respectively.
P-44.1.2.1 When two or more atoms denoting different classes are present in a compound, and when the choice for parent compound is between these atoms, the parent compound is the one belonging to the class cited first in the seniority of classes given above. A single senior atom is sufficient to give seniority to the parent hydride.
| This rule is broader than Rule D-1.34 in the 1979 Recommendations (ref. 1) that referred to compounds where the heteroatoms were directly attached to each other. |
|
Examples:
Si(CH3)4
tetramethylsilane (PIN)
(Si is senior to C)
CH3-PH-SiH3
methyl(silyl)phosphane (PIN)
(P is senior to Si or C)
Al(CH2-CH3)3
triethylalumane
(Al is senior to C)
HS-S-S-S-S-SiH2-SiH2-SiH3
1-pentasulfanyltrisilane (preselected name)
(Si is senior to S)
Note: The HS– group at the end of the sulfur chain is not cited as a suffix; see P-21.2.2.
H3Si-SiH2-CH2-CH2-PH2
(2-disilanylethyl)phosphane (PIN)
(P is senior to Si)
H2Sb-CH2-AsH2
(stibanylmethyl)arsane (PIN)
(As is senior to Sb)
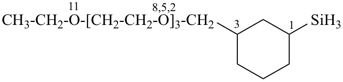
[3-(2,5,8,11-tetraoxatridecan-1-yl)cyclohexyl]silane (PIN)
(Si is senior to O, and both are senior to C)
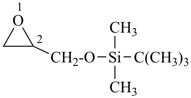
tert-butyldi(methyl)(oxiranylmethoxy)silane (PIN)
(Si is senior to O)
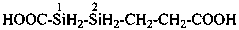
2-(2-carboxyethyl)disilane-1-carboxylic acid (PIN)
(Si is senior to C)
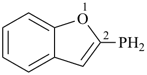
(1-benzofuran-2-yl)phosphane (PIN)
(P is senior to O)
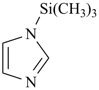
1-(trimethylsilyl)-1H-imidazole (PIN)
(N is senior to Si)
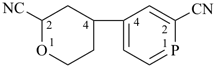
4-(2-cyanophosphinin-4-yl)oxane-2-carbonitrile (PIN)
(O > P; see P-44.2;
senior parent cannot be chosen by P-44.1.2)
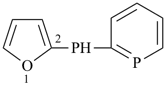
2-[(phosphinin-2-yl)phosphanyl]furan (PIN)
(P-ring is senior to P-chain; O-ring is senior to P-ring)
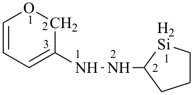
1-(2H-pyran-3-yl)-2-(silolan-2-yl)hydrazine (PIN)
( N > Si > O)
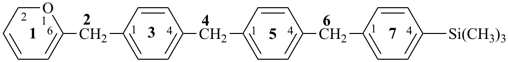
trimethyl[12H-1(6)-pyrana-3,5(1,4),7(1)-tribenzenaheptaphan-74-yl]silane (PIN)
(Si is senior to O)
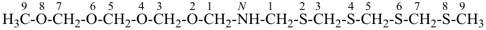
N-(2,4,6,8-tetrathianonan-1-yl)-2,4,6,8-tetraoxanonan-1-amine (PIN)
[O > S; see P-44.3;
senior parent cannot be chosen by P-44.1.2]
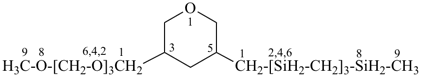
1-[5-(2,4,6,8-tetrasilanonan-1-yl)oxan-3-yl]-2,4,6,8-tetraoxanonane (PIN)
[O-chain > Si-chain (see P-44.3;
senior parent cannot be chosen by P-44.1.2)]
P-44.1.2.2 Systems composed of rings and chains (exclusive of linear phanes)
Two methods are recognized to name systems composed of rings and chains (exclusive of linear phanes).
(1) Within the same class, a ring or ring system has seniority over a chain. When a ring and a chain contain the same senior element, the ring is chosen as parent. Rings and chains are chosen regardless of their degree of hydrogenation. As a consequence, this approach prefers the choice of a ring over a chain in systems composed of cyclic and acyclic hydrocarbons.
(2) The context may favor the ring or the chain, so that, for example, substituents may be treated alike or an unsaturated acyclic structure may be recognized, or the one chosen has the greater number of skeletal atoms in the ring or in the principal chain of the acyclic structure.
In the examples that follow, when a choice is possible, names formed by both methods are given. For selection of a preferred IUPAC name, see P-52.2.8.
Examples:
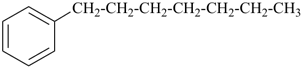
(1) heptylbenzene (PIN)
(ring is senior to chain)
(2) 1-phenylheptane
(chain has greater number of skeletal atoms)
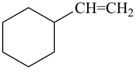
(1) ethenylcyclohexane (PIN)
(ring is senior to chain)
(2) cyclohexylethene
(emphasizes unsaturation)
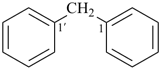
(1) 1,1′-methylenedibenzene (PIN)
(ring is senior to chain)
(2) diphenylmethane
(treats phenyl groups alike as substituents)
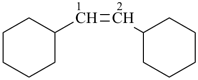
(1) 1,1′-(ethene-1,2-diyl)dicyclohexane (PIN)
(ring is senior to chain)
(2) 1,2-dicyclohexylethene
(emphasizes unsaturation)
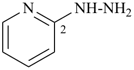
(1) 2-hydrazinylpyridine (PIN)
(ring is senior to chain)
(2) (pyridin-2-yl)hydrazine
(pyridin-2-yl)diazane
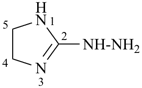
(1) 2-hydrazinyl-4,5-dihydro-1H-imidazole (PIN)
2-diazanyl-4,5-dihydro-1H-imidazole
(ring is senior to chain)
(2) (4,5-dihydro-1H-imidazol-2-yl)hydrazine
P-44.1.3 Seniority order only for rings and ring systems. Criteria that apply only when the choice for parent structure is between two or more rings or ring systems are given in P-44.2.
P-44.1.4 Seniority among acyclic chains (the principal chain). Criteria that apply only when the choice for parent structure is between two or more acyclic chains are given in P-44.3.
P-44.1.5 Criteria applicable to rings, ring systems, or acyclic chains, such as unsaturation, the presence of skeletal atoms with different bonding numbers, isotopically modified compounds, and stereochemical configurations are given in P-44.4.
P-44.2 SENIORITY ORDER ONLY FOR RINGS AND RING SYSTEMS
P-44.2.1 Criteria general to all rings and ring systems (other than phanes, both cyclic and linear, for which see P-44.2.2.2.2 and P-44.2.2.2.6, respectively)
P-44.2.2 Criteria specific to a particular kind of ring or ring system
P-44.2.1 If application of P-44.1 does not effect a choice, general criteria for determining ring seniority given below are applied successively until there are no alternatives remaining. These criteria are listed first and then separately illustrated in P-44.2.1.2 through P-44.2.1.8, below.
The senior ring or ring system:
(a) is a heterocycle;
(b) has at least one nitrogen atom;
(c) has at least one heteroatom (in the absence of nitrogen) that occurs earlier in the following sequence: F > Cl > Br > I > O > S > Se > Te > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
(d) has the greater number of rings;
(e) has the greater number of skeletal atoms;
(f) has the greater number of heteroatoms of any kind;
(g) has the greater number of heteroatoms occurring earlier in the sequence: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
P-44.2.1.1 General methodology.
In order to apply P-44.2 after P-44.1 has failed to reach a conclusion, there must not be a characteristic group present in the compound, or the same number of a characteristic group must be present in all cyclic structures to be compared. In the following examples, as well as examples throughout P-44.2, seniority is expressed by the symbol ‘>’ to be read as ‘senior to’. In the case of larger structures the phrase ‘is senior to’ is cited between the senior structure and the less senior structure.
Examples (the symbol > means ‘is senior to’):
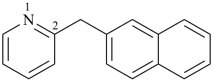
2-[(naphthalen-2-yl)methyl]pyridine (PIN)
(pyridine > naphthalene)
Note: In this compound, there is no characteristic group to be cited as suffix. One ring must be chosen as the senior ring that will serve as the parent hydride; the other ring will be cited as a prefix to the parent hydride. Criteria in P-44.2.1 must be applied starting from (a), in the order given, until a decision is reached. In this case, application of the first criterion (a) leads to a decision: the ring containing a nitrogen atom is selected as parent hydride; the naphthalene ring system is cited as a substituent.
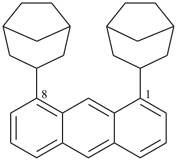
1,8-di(bicyclo[3.2.1]octan-3-yl)anthracene (PIN)
Note: In the above compound, there is no characteristic group to be cited as suffix. One ring must be chosen as the senior ring that will serve as the parent hydride; the other ring will be cited as a prefix to the parent hydride. Criteria in P-44.2.1 must be applied starting from criterion (a), in the order given, until a decision is reached. In this case, application of criterion (d) leads to a decision: anthracene has more rings and is the parent structure; the bicyclo ring is cited as a substituent.
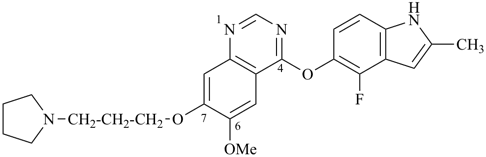
4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin- 1-yl)propoxy]quinazoline (PIN)
(quinazoline > indole > pyrrolidine)
Note: In this compound, there are two ring systems and one ring. No characteristic group to be cited as suffix is present. Characteristic groups to be cited as prefixes are ignored at this stage of the selection of the senior ring or ring system. The ring system ‘quinazoline’ is selected as parent hydride after applying criteria (a), (b), and (d) in P-44.2.1; and finally it is criterion (e) that permits a decision to be reached between ‘quinazoline’ and ‘indole’. Quinazoline is the parent hydride and both indole and pyrrolidine are included in the substituents cited as prefixes.
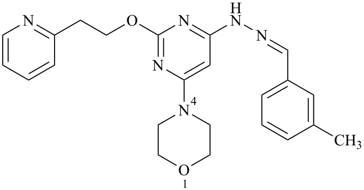
4-(6-{2-[(3-methylphenyl)methylidene]hydrazin-1-yl}-2-[2-(pyridin-2-yl)ethoxy]pyrimidin-4-yl)morpholine (PIN)
(morpholine > pyrimidine > pyridine > benzene)
Note: In this compound, ‘morpholine’ is chosen as parent hydride, as a decision in the selection of the senior ring cannot be made until criterion (g  ) in P-44.2.1 is reached. The rings benzene, pyridine, and pyrimidine are expressed within the substituent prefixes.
) in P-44.2.1 is reached. The rings benzene, pyridine, and pyrimidine are expressed within the substituent prefixes.
P-44.2.1.2 The senior ring or ring system is a heterocycle [criterion (a) in P-44.2.1]. Example (the symbol > means ‘is senior to’):
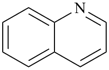 | > | 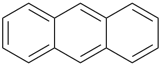 |
| quinoline (PIN) | | anthracene (PIN) |
P-44.2.1.3 The senior ring or ring system has at least one nitrogen ring atom [criterion (b) in P-44.2.1]
Examples (the symbol > means ‘is senior to’):
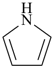 | > | 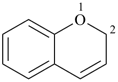 |
| 1H-pyrrole (PIN) | | 2H-1-benzopyran (PIN)
2H-chromene |
| |
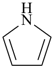 | > | 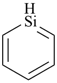 |
| 1H-pyrrole (PIN) | | 2H-siline (PIN) |
P-44.2.1.4 The senior ring or ring system has at least one heteroatom (in the absence of nitrogen) that occurs earlier in the following sequence: F > Cl > Br > I > O > S > Se > Te > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (c) in P-44.2.1].
Examples (the symbol > means ‘is senior to’):
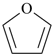 | > | 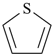 |
| furan (PIN) | | thiophene (PIN) |
| [O > S] |
| |
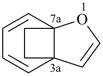 | > | 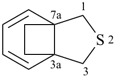 |
| 3a,7a-ethano-1-benzofuran (PIN) | | 1H,3H-3a,7a-ethano-2-benzothiophene (PIN) |
| [O > S] |
| |
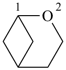 | > | 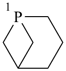 |
| 2-oxabicyclo[3.1.1]heptane (PIN) | | 1-phosphabicyclo[3.1.1]heptane (PIN) |
| [O > P] |
P-44.2.1.5 The senior ring or ring system has the greater number of rings [criterion (d) in P-44.2.1].
Example (the symbol > means ‘is senior to’):
 | > |  |
| isoquinoline (PIN) | | 1H-pyrrole (PIN) |
| [2 rings > 1 ring] |
P-44.2.1.6 The senior ring or ring system has the greater number of skeletal atoms [criterion (e) in P-44.2.1].
Examples (the symbol > means ‘is senior to’):
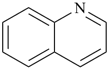 | > | 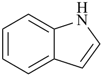 |
| quinoline (PIN) | | 1H-indole (PIN) |
| [10 skeletal atoms > 9 skeletal atoms] |
| |
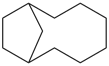 | > | 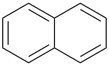 |
| bicyclo[6.2.1]undecane (PIN) | | naphthalene (PIN) |
| [11 skeletal atoms > 10 skeletal atoms] |
Note: Because of the hierarchical nature of these criteria, this criterion regarding the number of skeletal atoms supersedes P-44.2.2.2, which prefers a fused ring to a bridged fused ring.
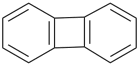 | > |  |
| biphenylene (PIN) | | 1,4-methanonaphthalene (PIN) |
| [12 skeletal atoms > 11 skeletal atoms] |
Note: Because of the hierarchical nature of these criteria, this criterion regarding the number of skeletal atoms supersedes P-44.2.2.2, which prefers a fused ring to a bridged fused ring.
P-44.2.1.7 The senior ring or ring system has the greater number of heteroatoms of any kind [(criterion (f) in P-44.2.1]
Examples (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| 2,5,7-trioxabicyclo[4.1.1]octane (PIN) | | 2-oxabicyclo[4.1.1]octane (PIN) |
| [3 heteroatoms > 1 heteroatom] |
| |
| (2) | 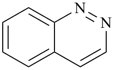 | > | 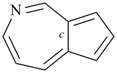 |
| cinnoline (PIN) | | cyclopenta[c]azepine (PIN) |
| [2 heteroatoms > 1 heteroatom] |
| |
| (3) |  | > |  |
| 5,6,11,12-tetraoxadispiro[3.2.37.24]dodecane (PIN) | | 11-oxadispiro[3.1.36.34]dodecane (PIN) |
| [4 heteroatoms > 1 heteroatom] |
| |
| (4) |  | > | 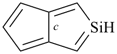 |
| silolo[3,4-c]silole (PIN) | | cyclopenta[c]silole (PIN) |
| [2 heteroatoms > 1 heteroatom] |
| |
| (5) | 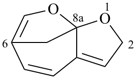 | > | 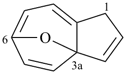 |
| 2H-6,8a-methanofuro[2,3-b]oxepine (PIN) | | 1H-3a,6-epoxyazulene (PIN) |
| [2 heteroatoms > 1 heteroatom] |
P-44.2.1.8 The senior ring or ring system has the greater number of heteroatoms that occur earlier in the following sequence: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (g) in P-44.2.1].
Examples (the symbol > means ‘is senior to’):
| (1) | 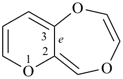 | > | 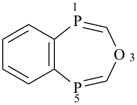 |
| pyrano[3,2-e][1,4]dioxepine (PIN) | | 3,1,5-benzoxadiphosphepine (PIN) |
| [3 heteroatoms = 3 heteroatoms; 3 oxygen atoms > 1 oxygen atom] |
| |
| (2) | 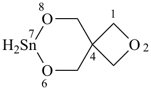 | > | 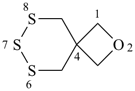 |
| 2,6,8-trioxa-7-stannaspiro[3.5]nonane (PIN) | | 2-oxa-6,7,8-trithiaspiro[3.5]nonane (PIN) |
| [4 heteroatoms = 4 heteroatoms; 3 oxygen atom > 1 oxygen atom] |
| |
| (3) | 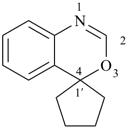 | > | 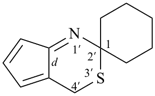 |
| spiro[[3,1]benzoxazine-4,1′-cyclopentane] (PIN) | | 4′H-spiro[cyclohexane-1,2′-cyclopenta[d][1,3]thiazine] (PIN) |
| [2 heteroatoms = 2 heteroatoms]
[1 nitrogen atom, 1 oxygen atom > 1 nitrogen atom, 1 sulfur atom] |
P-44.2.2 Seniority criteria for determining ring seniority applicable to particular types of ring systems.
P-44.2.2.1 Monocycles (see P-22)
If P-44.2.1 does not provide a decision, further criteria applicable to monocyclic rings are found in P-44.4.
P-44.2.2.2 Polycyclic systems. The senior polycyclic ring system occurs first in the following list of polycyclic ring system types:
| The seniority order between parent hydrides having the same number of identical heteroatoms, the same number of rings, and the same number of skeletal atoms is a change from previous practice. Seniority of polycyclic ring systems is now facilitated by a hierarchical order of ring systems, which includes cyclic and acyclic phane systems and ranks all ring systems in the order given in this recommendation. |
|
(a) spiro ring system (see P-24);
(b) cyclic phane system (see P-26);
(c) fused ring system (see P-25);
(d) bridged fused ring system (see P-25);
(e) nonfused bridged ring system (see P-23);
(f) linear phane system (see P-26);
(g) ring assembly (see P-28).
Choices within each type are illustrated in P-44.2.2.2.1 through P-44.2.2.2.7.
Further criteria applicable to polycyclic ring systems are found in P-44.4.
Examples (the symbol > means ‘is senior to’):
| (1) | 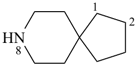 | > |  |
| 8-azaspiro[4.5]decane (PIN) | | quinoline (PIN) |
| [spiro ring system (a) > fused ring system (c)] |
| |
| (2) |  | > | 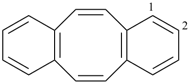 |
| 1,4(1,4)-dibenzenacyclohexaphane (PIN) | | dibenzo[a,e][8]annulene (PIN) |
| [cyclic phane ring system (b) > fused ring system (c)] |
| |
| (3) | 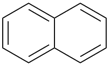 | > | 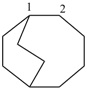 |
| naphthalene (PIN) | | bicyclo[4.2.2]decane (PIN) |
| [fused ring system (c) > nonfused bridged ring system (e)] |
| |
| (4) | 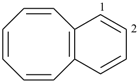 | > | 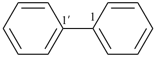 |
| benzo[8]annulene (PIN) | | 1,1′-biphenyl (PIN) |
| [fused ring system (c) > ring assembly (g)] |
P-44.2.2.2.1 Seniority criteria for spiro ring systems given below are applied successively until no alternatives remain. These criteria are illustrated in P-44.2.2.2.1.1 through P-44.2.2.2.1.3. The senior spiro system:
(a) has the greater number of spiro fusions;
(b) consists of saturated monocyclic rings;
(c) consists of only discrete components.
Further criteria applicable to spiro ring systems are found in P-44.4.
P-44.2.2.2.1.1 The senior spiro system has the greater number of spiro fusions [criterion (a) in P-44.2.2.2.1].
Example (the symbol > means ‘is senior to’):
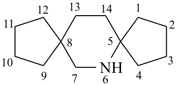 | > |  |
| 6-azadispiro[4.2.48.25]tetradecane (PIN) | | 2′H-spiro[cyclopentane-1,1′-isoquinoline] (PIN) |
| [2 spiro fusions > 1 spiro fusion] |
P-44.2.2.2.1.2 The senior spiro system consists of only saturated monocyclic rings [criterion (b) in P-44.2.2.2.1] and has the lower locant(s) for spiro atom(s);
Example (the symbol > means ‘is senior to’):
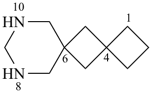 | > | 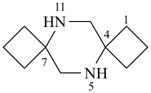 |
| 8,10-diazadispiro[3.1.56.14]dodecane (PIN) | | 5,11-diazadispiro[3.2.37.24]dodecane (PIN) |
[saturated monocyclic rings each with 2 spiro fusions;
the locant set for spiro fusions ‘4,6’ is lower that ‘4,7’] |
P-44.2.2.2.1.3 The senior spiro system consists only of discrete components [criterion (c) in P-44.2.2.2.1] and:
(a) has the senior component determined by criteria above and below for the appropriate type of ring or ring system when the components are compared in their order of seniority;
Example (the symbol > means ‘is senior to’):
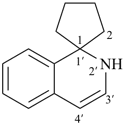
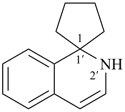 | > |  |
| 2′H-spiro[cyclopentane-1,1′-isoquinoline] (PIN) | | spiro[indene-1,4′-piperidine] (PIN) |
| [isoquinoline > piperidine] |
(b) has the senior component determined by criteria above and below for the appropriate kind of ring or ring system when compared in their order of citation in the name;
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
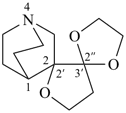
(I) 1′-azadispiro[[1,3]dioxolane-2,2′-bicyclo[2.2.2]octane-5′,2′′-oxolane] (PIN)
is senior to
(II) 4-azadispiro[bicyclo[2.2.2]octane-2,2′-oxolane-3′,2′′-[1,3]dioxolane] (PIN)
[first cited component dioxolane > bicyclo[2.2.2]octane] |
(c) has the lower locant(s) for the spiro atoms in the order of citation in the name.
Example (the symbol > means ‘is senior to’):
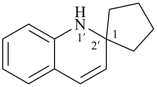 | > | 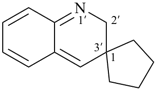 |
| (I) | | (II) |
(I) 1′H-spiro[cyclopentane-1,2′-quinoline] (PIN)
is senior to
(II) 2′H-spiro[cyclopentane-1,3′-quinoline] (PIN)
[spiro fusion locant set 1,2′ in (I) is lower than 1,3′ in (II)] |
P-44.2.2.2.2 Seniority criteria for the cyclic phane systems given below are applied successively until no alternatives remain. These criteria are illustrated in P-44.2.2.2.2.1 through P-44.2.2.2.2.8.
The senior cyclic phane system:
(a) is the one occurring earlier in the following list of basic phane skeletal ring systems: spiro, von Baeyer, monocyclic;
(b) has the senior amplificant, as defined by P-44.2.1.2 through P-44.2.1.8;
(c) has the lower superatom locant(s) for all amplificants, first as a set compared term by term in order of increasing value and then in order of citation in the name;
(d) has the lower locant(s) for senior amplificants;
(e) has the lower set of attachment locants considered as a set when compared term by term in order of increasing numerical value;
(f) has the lower set of attachment locants when compared term by term in their order of citation in the name;
(g) has the lower locant(s) for heteroatoms introduced by skeletal replacement (‘a’) nomenclature without regard to kind;
(h) has the lower locant(s) for heteroatoms introduced by skeletal replacement (‘a’) nomenclature first cited in the following order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
Further criteria applicable to cyclic phane systems are found in P-44.4.
P-44.2.2.2.2.1 The senior cyclic phane system occurs earlier in the following list of basic phane skeletal ring systems: spiro > nonfused bridged (von Baeyer) > monocyclic.
Examples (in order of decreasing seniority according to the criteria (a) in P-44.2.2.2.2):
4,12(1,3)-dibenzenaspiro[7.7]pentadecaphane (PIN)
(a spiro phane skeletal system)
is senior to
5,12(1,3)-dibenzenabicyclo[7.5.1]pentadecaphane (PIN)
(a von Baeyer phane skeletal system)
is senior to
1(1,3)-benzena-5(1,3)-cyclohexana-3(1,3)-cyclopentanacycloundecaphane (PIN)
(a monocyclic phane skeletal system)
P-44.2.2.2.2.2 The senior cyclic phane system has the senior amplificant, as defined by P-44.2.1.2 through P-44.2.1.8 [criterion (b) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 1(2,6)-pyrazina-6(1,3)-benzenacyclodecaphane (PIN)
is senior to
(II) 1,6(2,4)-dipyridinacyclodecaphane (PIN)
[pyrazine > pyridine] |
P-44.2.2.2.2.3 The senior cyclic phane system has the lower superatom locant(s) for all amplificants, first as a set compared term by term in order of increasing numerical value and then in order of their citation in the name [criterion (c) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
| 1,4(1,3)-dibenzenacyclononaphane (PIN) | | 1,5(1,3)-dibenzenacyclononaphane (PIN) |
| [superatom locant set ‘1,4’ in (I) is lower than ‘1,5’ in (II)] |
P-44.2.2.2.2.4 The senior cyclic phane system has the lower locants for senior amplificants [criterion (d) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 3(3,5)-pyridina-1(1,3),6(1,4)-dibenzenacyclotridecaphane (PIN)
is senior to
(II) 6(2,5)-pyridina-1,3(1,3)-dibenzenacyclotridecaphane (PIN)
[superatom locant ‘3’ for the senior amplificant pyridine is lower than ‘6’] |
P-44.2.2.2.2.5 The senior cyclic phane system has the lower set of attachment locants considered as a set when compared term by term in order of increasing numerical value [criterion (e) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
| 1,6(1,3)-dibenzenacyclodecaphane (PIN) | | 1(1,3),6(1,4)-dibenzenacyclodecaphane (PIN) |
| [the attachment locant set ‘(1,3)(1,3)’ is lower than ‘(1,3)(1,4)’] |
P-44.2.2.2.2.6 The senior cyclic phane system has the lower set of attachment locants when compared term by term in their order of citation in the name [criterion (f) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 1,3,5,7(2,4)-tetrapyridinacyclooctaphane (PIN)
is senior to
(II) 1,5(2,4),3,7(4,2)-tetrapyridinacyclooctaphane (PIN)
[the attachment locant set ‘(2,4)(2,4)(2,4)(2,4)’ is lower than ‘(2,4)(2,4)(4,2)(4,2)’] |
P-44.2.2.2.2.7 The senior cyclic phane system has the lower locant(s) for heteroatoms specified by skeletal replacement (‘a’) prefixes without regard to kind [criterion (g) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 3-oxa-2-thia-1,7(1,3)-dibenzenacyclododecaphane (PIN)
is senior to
(II) 5-oxa-2-thia-1,7(1,3)-dibenzenacyclododecaphane (PIN)
[the heteroatom locant set ‘2,3’ is lower than ‘2,5’] |
P-44.2.2.2.2.8 The senior cyclic phane system has the lower locant(s) for heteroatoms specified by skeletal replacement (‘a’) prefixes first cited in the following order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (h) in P-44.2.2.2.2].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 2-oxa-3-thia-1,7(1,3)-dibenzenacyclododecaphane (PIN)
is senior to
(II) 3-oxa-2-thia-1,7(1,3)-dibenzenacyclododecaphane (PIN)
[the locant ‘2’ for the senior heteroatom ‘O’ is lower than ‘3’] |
P-44.2.2.2.3 Seniority criteria for fused ring systems given below are applied successively until no alternatives remain. The criteria are illustrated in P-44.2.2.2.3.1 through P-44.2.2.2.3.5. The senior fused ring system:
(a) has the larger individual ring component at first point of difference when their ring sizes are compared in order of decreasing size;
(b) has the greater number of rings in a horizontal row;
(c) has the lower letter(s) in the fusion descriptor when compared as a set; letters omitted in names are considered in the application of this criterion;
(d) has the lower number(s) in the fusion descriptor, in the order of appearance in the name; locants omitted in names are considered in application of this criterion;
(e) has the senior ring system component according to P-25.8 when its components are compared in order of decreasing seniority.
Further criteria applicable to fused ring systems are found in P-44.4.
P-44.2.2.2.3.1 The senior fused ring system has the larger individual ring component at first point of difference when their ring sizes are compared in order of decreasing size [criterion (a) in P-44.2.2.2.3].
Example (the symbol > means ‘is senior to’):
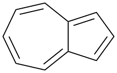 | > | 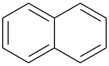 |
| azulene (PIN) | | naphthalene (PIN) |
| [the ring size ‘7’ in the ring set ‘7,5’ is larger than ‘6’ in the ring set ‘6,6’] |
P-44.2.2.2.3.2 The senior fused ring system has the greater number of rings in a horizontal row [criterion (b) in P-44.2.2.2.3].
Example (the symbol > means ‘is senior to’):
| (1) | 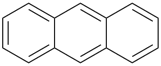 | > | 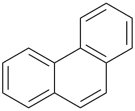 |
| anthracene (PIN) | | phenanthrene (PIN) |
| [three rings in the horizontal row is greater than two] |
| |
| (2) | 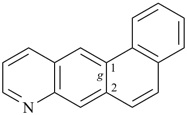 | > | 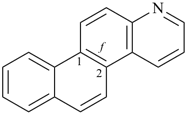 |
| naphtho[1,2-g]quinoline (PIN) | | naphtho[2,1-f]quinoline (PIN) |
| [three rings in the horizontal row is greater than two] |
P-44.2.2.2.3.3 The senior fused ring system has the lower letter(s) alphabetically in the fusion descriptor when compared as a set; letters omitted in names are considered in the application of this criterion [criterion (c) in P-44.2.2.2.3].
Example (the symbol > means ‘is senior to’):
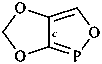 | > | 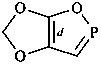 |
| 5H-[1,3]dioxolo[4,5-c][1,2]oxaphosphole (PIN) | | 5H-[1,3]dioxolo[4,5-d][1,2]oxaphosphole (PIN) |
| [the letter ‘c’ in the fusion descriptor is lower alphabetically than ‘d’] |
P-44.2.2.2.3.4 The senior fused ring system has the lower set of numbers in the fusion descriptor, in the order of appearance in a name; locants omitted in names are taken into consideration in application of this criterion [criterion (d) in P-44.2.2.2.3].
Example (the symbol > means ‘is senior to’):
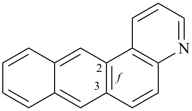
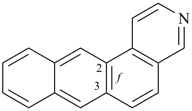
 | > |  |
| naphtho[1,2-f]quinoline (PIN) | | naphtho[2,1-f]quinoline (PIN) |
| [the locant set ‘1,2’ in the fusion descriptor is lower than ‘2,1’] |
P-44.2.2.2.3.5 The senior fused ring system has the senior ring system component according to P-25.8 when its components are compared in order of decreasing seniority [criterion (e) in P-44.2.2.2.3].
Example (the symbol > means ‘is senior to’):
 | > |  |
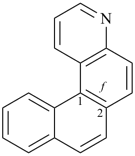
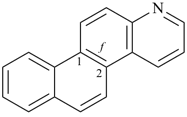
| naphtho[2,3-f]quinoline (PIN) | | naphtho[2,3-f]isoquinoline (PIN) |
| [the senior ring system quinoline is senior to isoquinoline (see P-25.2.1, Table 2.8)] |
P-44.2.2.2.4 Seniority criteria for bridged fused ring systems given below are applied successively until no alternatives remain. The criteria are illustrated in P-44.2.2.2.4.1 through P-44.2.2.2.4.14. The senior bridged fused ring system:
(a) has the bridged ring system with the greater number of rings before bridging;
(b) is the bridged ring system with the greater number of ring atoms before bridging;
(c) is the bridged ring system with the fewer heteroatoms in the fused ring system before bridging;
(d) is the bridged ring system with the senior fused ring system before bridging according to P-44-2.2.2.3;
(e) has the lower set of locants for bridge attachments;
(f) has the lower locant(s) for heteroatoms in bridges, without regard to kind;
(g) has the lower locant(s) for heteroatoms in bridges, in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
(h) has the fewer composite bridges;
(i) has the fewer dependent bridges;
(j) has fewer atoms in dependent bridges;
(k) has the greater number of divalent bridges;
(l) has the lower set of locants for attachment of independent bridges;
(m) has the lower set of locants for attachment of dependent bridges;
(n) has the fused ring system with the greater number of noncumulative double bonds before bridging.
Further criteria applicable to bridged fused ring systems are found in P-44.4.
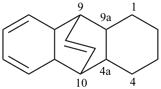
P-44.2.2.2.4.1 The senior bridged fused ring system has the bridged ring system with the greater number of rings before bridging [criterion (a) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
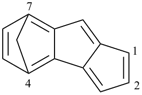 | > |  |
| 4,7-methanocyclopenta[a]indene (PIN) | | 2H-1,4:5,8-dimethanobenzo[7]annulene (PIN) |
| [three rings in the ring system before bridging is greater than two] |
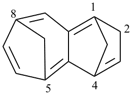
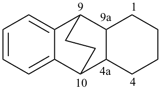
P-44.2.2.2.4.2 The senior bridged fused ring system is the bridged ring system with the greater number of ring atoms before bridging [criterion (b) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
 | > |  |
| 1H-3,10a-methanophenanthrene (PIN) | | 1,4a-ethanofluorene (PIN) |
| [fourteen ring atoms in the ring system before bridging is greater than thirteen] |
P-44.2.2.2.4.3 The senior bridged fused ring system is the bridged ring system with the fewer heteroatoms in the fused ring system before bridging [criterion (c) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
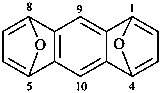 | > | 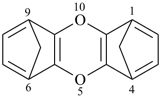 |
| 1,4:5,8-diepoxyanthracene (PIN) | | 1,4:6,9-dimethanooxanthrene (PIN) |
| [zero heteroatoms in the fused ring system before bridging is fewer than two] |
P-44.2.2.2.4.4 The senior bridged fused ring system is the bridged ring system with the senior fused ring system before bridging according to P-44.2.2.2.3 [criterion (d) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
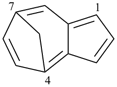 | > | 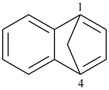 |
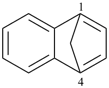
| 4,7-methanoazulene (PIN) | | 1,4-methanonaphthalene (PIN) |
| [azulene is senior to naphthalene, see P-44.2.1] |
P-44.2.2.2.4.5 The senior bridged fused ring system has the lower set of locants for bridge attachments [criterion (e) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
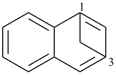 | > |  |
| 1,3-methanonaphthalene (PIN) | | 1,4-methanonaphthalene (PIN) |
| [the locant set for bridge attachments ‘1,3’ is lower than ‘1,4’] |
P-44.2.2.2.4.6 The senior bridged fused ring system has the lower locant(s) for heteroatoms in bridges, without regard to kind [criterion (f) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
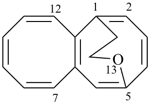 | > | 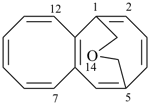 |
| 5,1-(epoxyethano)octalene (PIN) | | 1,5-(methanooxymethano)octalene (PIN) |
| [the locant ‘13’ for the bridge oxygen atom is lower than ‘14’] |
P-44.2.2.2.4.7 The senior bridged fused ring system has the lower locants for heteroatoms in bridges, in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (g) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
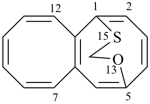 | > | 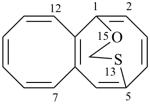 |
| 5,1-(epoxymethanosulfano)octalene (PIN) | | 1,5-(epoxymethanosulfano)octalene (PIN) |
| [the locant ‘13’ for the senior oxygen atom in the bridge is lower than ‘15’] |
P-44.2.2.2.4.8 The senior bridged fused ring system has the fewer composite bridges [criterion (h) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
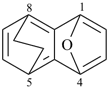 | > | 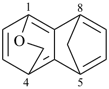 |
| 1,4-epoxy-5,8-ethanonaphthalene (PIN) | | 1,4-(epoxymethano)-5,8-methanonaphthalene (PIN) |
| [zero composite bridges is fewer than one] |
P-44.2.2.2.4.9 The senior bridged fused ring system has the fewer dependent bridges [criterion (i) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
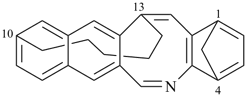 | > |  |
| (I) | | (II) |
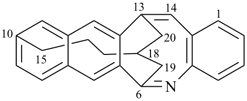
(I) 1,4-methano-10,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
is senior to
(II) 13,18-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
[zero dependent bridges is fewer than one] |
P-44.2.2.2.4.10 The senior bridged fused ring system has fewer atoms in dependent bridges [criterion (j) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
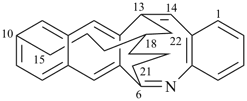 | > | 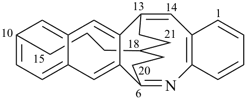 |
| (I) | | (II) |
(I) 13,18-methano-6,10-heptanonaphtho[2,3-c][1]benzazocine (PIN)
is senior to
(II) 13,18-ethano-6,10-hexanonaphtho[2,3-c][1]benzazocine (PIN)
[one atom in the dependent bridge in (I) (atom 22)
is fewer the two
atoms in (II) (atoms 21 and 22)] |
P-44.2.2.2.4.11 The senior bridged fused ring system has the greater number of divalent bridges [criterion (k) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 1-oxa-2,7:6,8-dimethanocycloocta[1,2,3-cd]pentalene (PIN)
is senior to
(II) 1-oxa-5,9,2-(ethane[1,1,2]triyl)cycloocta[1,2,3-cd]pentalene (PIN)
[‘2’ divalent bridges in (I) is senior to the ‘1’ trivalent bridge in (II)] |
P-44.2.2.2.4.12 The senior bridged fused ring system has the lower set of locants for attachment of independent bridges [criterion (l) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
(I) 13,18-methano-6,9-pentanonaphtho[2,3-c][1]benzazocine (PIN)
is senior to
(II) 13,18-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
[the locant set ‘6,9’ for the independent bridge in (I) is lower than the locant set ‘6,10’ in (II)] |
P-44.2.2.2.4.13 The senior bridged fused ring system has the lower set of locants for attachment of dependent bridges [criterion (m) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
 | > | 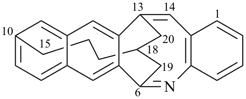 |
| (I) | | (II) |
13,17-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN) (I)
is senior to
13,18-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN) (II)
[the locant set ‘13,17’ for the dependent bridge in (I)
is lower
than the locant set ‘13,18’ in (II)] |
P-44.2.2.2.4.14 The senior bridged fused ring system has the fused ring system with the greater number of noncumulative double bonds before bridging [criterion (n) in P-44.2.2.2.4].
Example (the symbol > means ‘is senior to’):
 | > |  |
| (I) | | (II) |
1,2,3,4,4a,9,9a,10-octahydro-9,10-ethanoanthracene (PIN) (I)
is senior to
1,2,3,4,4a,8a,9,9a,10,10a-decahydro-9,10-ethenoanthracene (PIN) (II)
[three noncumulative double bonds in (I) is greater than the
two noncumulative double bonds in (II)] |
P-44.2.2.2.5 Seniority criteria for bridged nonfused ring systems (von Baeyer ring systems) are applied successively until there are no alternatives remaining. These criteria are illustrated in P-44.2.2.2.5.1 through P-44.2.2.2.5.3. The senior bridged nonfused ring system:
(a) has the lower number at the first point of difference in the descriptor set for describing ring sizes when considered in order of citation in the name;
(b) has the lower set of bridge attachment locants (superscript locants) at the first point of difference when compared term by term in order of increasing numerical value;
(c) has the lower set of bridge attachment locants (superscript locants) at first point of difference when compared term by term in their order of citation in the name.
Further criteria applicable to bridged nonfused ring systems are found in P-44.4.
P-44.2.2.2.5.1 The senior bridged nonfused ring system has the lower number in the descriptor set for describing ring sizes at the first point of difference when considered in order of citation in the name [criteria (a) in P-44.2.2.2.5].
Example (the symbol > means ‘is senior to’):
 | > |  |
| bicyclo[2.2.2]octane (PIN) | | bicyclo[3.2.1]octane (PIN) |
| [the ring descriptor set ‘2,2,2’ is lower than ‘3,2,1’] |
P-44.2.2.2.5.2 The senior bridged nonfused ring system has the lower set of bridge attachment locants (superscript locants) at the first point of difference when compared term by term in order of increasing numerical value [criteria (b) in P-44.2.2.2.5].
Example (the symbol > means ‘is senior to’):
 | > |  |
| tricyclo[5.2.1.11,4]undecane (PIN) | | tricyclo[5.2.1.12,5]undecane (PIN) |
| [the bridge attachment locant set ‘1,4’ is lower than ‘2,5’] |
P-44.2.2.2.5.3 The senior bridged nonfused ring system has the lower set of bridge attachment locants (superscript locants) at first point of difference when compared term by term in their order of citation in the name [criterion (c) in P-44.2.2.2.5].
Example (the symbol > means ‘is senior to’):
 | > |  |
| tetracyclo[5.5.2.22,5.18,12]heptadecane (PIN) | | tetracyclo[5.5.2.22,6.18,12]heptadecane (PIN) |
| [the bridge attachment locant set ‘2,5,8,12’ is lower than ‘2,6,8,12’] |
P-44.2.2.2.6 Linear (acyclic) phanes
Although linear phane systems can be viewed as heteroacyclic chains, in which the amplificants are heteroatoms and the connecting atoms or chains are carbon atoms, seniority for linear (acyclic) phanes follows closely the criteria used for cyclic phane systems (see P-44.2.2.2.2). Accordingly, the seniority criteria for linear phane systems given below are applied successively until no alternatives remain. These criteria are illustrated in P-44.2.2.2.6.1 through P-44.2.2.2.6.10. The senior linear phane system has:
(a) the senior amplificant, as defined by P-44.2.1.2 through P-44.2.1.8;
(b) most amplificants in their order of seniority as defined by P-44.2.1.2 through P-44.2.1.8;
(c) the maximum number of skeletal nodes;
(d) the lower superatom locant(s) for the senior amplificant;
(e) the lower superatom locant(s) for all amplificants as a set when compared term by term in order of increasing value and then in order of citation in the name;
(f) the lower superatom locant(s) for all amplificants when compared in order of citation in the name;
(g) the lower set of attachment locants considered as a set when compared term by term in order of increasing numerical value;
(h) the lower set of attachment locants when compared term by term in their order of citation in the name;
(i) the greater number of heteroatoms introduced by skeletal replacement (‘a’) nomenclature without regard to kind;
(j) the greater number of heteroatoms introduced by skeletal replacement (‘a’) nomenclature first cited in the following order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
Further criteria applicable to linear (acyclic) phanes are found in P-44.4.
P-44.2.2.2.6.1 The senior linear phane system has the senior amplificant, as defined by P-44.2.1.2. through P-44.2.1.8 [criterion (a) in P-44.2.2.2.6]
Examples:
| (1) | 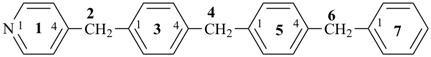 |
| 1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| is senior to |
|  |
| 1(4)-silina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| [the amplificant ‘pyridina’ is senior to the amplificant ‘silina’] |
| |
| (2) |  |
| 1(2)-furana-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| is senior to |
|  |
| 1(2)-thiophena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| [the amplificant ‘furana’ is senior to the amplificant ‘thiophena’] |
| |
| (3) |  |
| 1(2)-quinolina-7(4)-pyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| is senior to |
|  |
| 1,7(4)-dipyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| [the amplificant ‘quinolina’ is senior to the amplificant ‘pyridina’] |
| |
| (4) |  |
| 11H-1(2)-azepina-7(4)-pyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| is senior to |
| 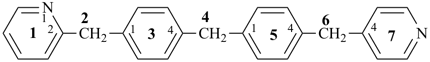 |
| 1(2),7(4)-dipyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| [the amplificant ‘azepina’ is senior to the amplificant ‘pyridina’] |
P-44.2.2.2.6.2 The senior linear phane system has the most amplificants in their order of seniority as defined by P-44.2.1.2 through P-44.2.1.8 [criterion (b) in P-44.2.2.2.6].
Examples:
| (1) | 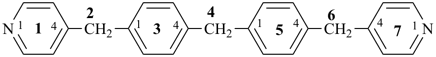 |
| 1,7(4)-dipyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| is senior to |
| 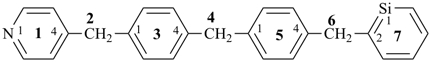 |
| 1(4)-pyridina-7(2)-silina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| [the amplificants ‘pyridina/pyridina’ are senior to the amplificants ‘pyridina/silina’] |
| |
| (2) | 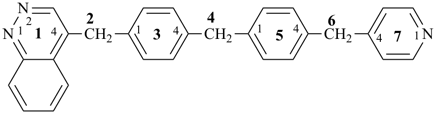 |
| 1(4)-cinnolina-7(4)-pyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| is senior to |
| 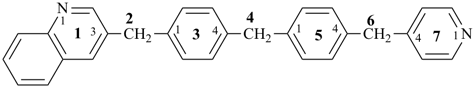 |
| 1(3)-quinolina-7(4)-pyridina-3,5(1,4)-dibenzenaheptaphane (PIN) |
| [the amplificants ‘cinnolina/pyridina’ are senior to the amplificants ‘quinolina/pyridina’] |
| |
| (3) | 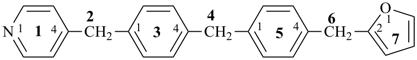 |
| 1(4)-pyridina-7(2)-furana-3,5(1,4)-dibenzenaheptaphane (PIN) |
| is senior to |
| 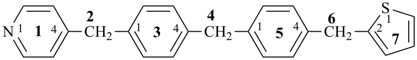 |
| 1(4)-pyridina-7(2)-thiophena-3,5(1,4)-dibenzenaheptaphane (PIN) |
| [the amplificants ‘pyridina/furana’ are senior to ‘pyridina/thiophena’] |
P-44.2.2.2.6.3 The senior linear phane system has the maximum number of skeletal nodes [criterion (c) in P-44.2.2.2.6]
Example:
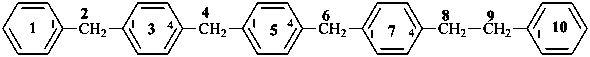
1,10(1),3,5,7(1,4)-pentabenzenadecaphane (PIN)
is senior to
1,9(1),3,5,7(1,4)-pentabenzenanonaphane (PIN)
[the decaphane has more nodes than the nonaphane]
P-44.2.2.2.6.4 The senior linear phane system has the lower superatom locant(s) for the senior amplificants [criterion (d) in P-44.2.2.2.6].
Example:
1(4),7(2,5)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
is senior to
1(4),8(2,5)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
[the locant set ‘1,7’ for the pyridina amplificants is lower than the set ‘1,8’]
P-44.2.2.2.6.5 The senior linear phane system has the lower superatom locant(s) for all amplificants as a set when compared term by term in order of increasing seniority [criterion (e) in P-44.2.2.2.6].
Example:
1,11(1),3,5,7(1,4)-pentabenzenaundecaphane (PIN)
is senior to
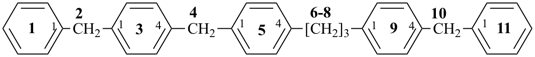
1,11(1),3,5,9(1,4)-pentabenzenaundecaphane (PIN)
[the locant set ‘1,3,5,7,11’ for the amplificants is lower than the set ‘1,3,5,9,11’]
P-44.2.2.2.6.6 The senior linear phane system has the lower superatom locant(s) for all amplificants when compared in order of citation in the name [criterion (f) in P-44.2.2.2.6].
Example:
1(4)-pyridina-7(2,5)-furana-9(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
is senior to
1(4)-pyridina-9(2,5)-furana-7(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
[the locant set for the amplificants in order of occurrence in
the name ‘1,7,9,3,5,11’ is lower than the locant set ‘1,9,7,3,5,11’]
P-44.2.2.2.6.7 The senior linear phane system has the lower set of attachment locants considered as a set when compared term by term in order of increasing numerical value [criterion (g) in P-44.2.2.2.6].
Example:
1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN)
is senior to
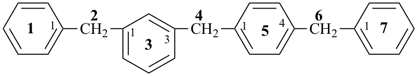
1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane (PIN)
[the locant set ‘1,1,1,1,3,3’ for amplificant attachment locants in increasing numerical order is lower than ‘1,1,1,1,3,4’]
P-44.2.2.2.6.8 The senior linear phane system has the lower set of attachment locants when compared term by term in their order of citation in the name [criterion (h) in P-44.2.2.2.6].
Example:
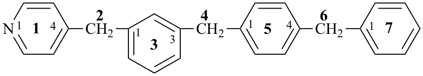
1(4)-pyridina-3(1,3),5(1,4),7(1)-tribenzenaheptaphane (PIN)
is senior to
1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane (PIN)
[the locant set ‘4,1,3,1,4,1’ for the amplificant locants in order of their citation in the name is lower than ‘4,1,4,1,3,1’]
P-44.2.2.2.6.9 The senior linear phane system has the greater number of heteroatoms introduced by skeletal replacement (‘a’) nomenclature without regard to kind [criterion (i) in P-44.2.2.2.6].
Example:
6-oxa-2-thia-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
is senior to
2-thia-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
[two heteroatoms intoduced by skeletal replacement (‘a’) nomenclature are senior to one such heteroatom]
P-44.2.2.2.6.10 The senior linear phane system has the greater number of heteroatoms introduced by skeletal replacement (‘a’) nomenclature first cited in the following order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (j) in P-44.2.2.2.6]
Example:
2,10-dioxa-1,11(4)-dipyridina-3,5,9(1,4)-tribenzenaundecaphane (PIN)
is senior to
2-oxa-10-thia-1,11(4)-dipyridina-3,5,9(1,4)-tribenzenaundecaphane (PIN)
[two oxygen atoms are senior to one oxygen atom and one sulfur atom]
P-44.2.2.2.7 Seniority criteria for ring assemblies are based on the appropriate criteria for rings and ring systems given in P-44.2.1. Phane nomenclature is used when two ring assemblies are linked together by atoms or chains to generate a system with at least seven nodes and two terminal rings or ring systems.
Example:
4-oxa-1(2),2,3(2,6)-tripyridina-5,6(1,4),7(1)-tribenzenaheptaphane (PIN)
16-([11,21:24,31]terphenyl]-14-yloxy)-12,22:26,32-terpyridine;
[criteria for
phane nomenclature (see P-52.2.5.1) are met for the PIN]
The following rules for seniority of ring assemblies are applied successively until there are no alternatives remaining:
(a) rings containing any heteroatom;
(b) rings containing nitrogen;
(c) in the absence of nitrogen, rings containing at least one heteroatom first cited in the following sequence; F > Cl > Br > I > O > S > Se > Te > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
(d) the greater number of rings;
(e) the greater number of atoms;
(f) the greater number of heteroatoms of any kind;
(g) the greater number of heteroatoms first cited in the following sequence: F > Cl > Br > I > O > S > Se >Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
Further criteria applicable to ring assemblies are found in P-44.4.
P-44.2.2.2.7.1 The senior ring assembly is composed of rings containing any heteroatom [criterion (a) in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
2,2′-biphosphinine (PIN)
| | 11,21:24,31-terphenyl (PIN)
1,1′:4′,1′′-terphenyl |
P-44.2.2.2.7.2 The senior ring assembly is composed of rings containing nitrogen [criterion (b) in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
2,2′-bipyridine (PIN)
| | 2H,2′H-2,2′-bipyran (PIN) |
P-44.2.2.2.7.3 The senior ring assembly in the absence of nitrogen is composed of rings containing at least one hetero atom first cited in the following sequence: F > Cl > Br > I > O > S > Se >Te > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (c) in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
| 2H,2′H-2,2′-bipyran (PIN) | | 2H,2′H-2,2′-bithiopyran (PIN) |
| [one oxygen atom per ring is preferred to one sulfur atom per ring] |
P-44.2.2.2.7.4 The senior ring assembly is composed of the greater number of rings [criterion (d) in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
2,2′-biquinoline (PIN)
| | 12,22:25,32-terpyridine (PIN)
2,2′:5′,2′′-terpyridine |
| [a ring system consisting of two rings is senior to a ring system consisting of one ring] |
P-44.2.2.2.7.5 The senior ring assembly is composed of the greater number of atoms [criterion (e) in P-44.2.2.2.7.]
Example (the symbol > means ‘is senior to’):
 | > |  |
1H,1′H-2,2′-biazepine (PIN)
| | 2,4′-bipyridine (PIN) |
| [seven atoms per ring is preferred to six atoms per ring] |
P-44.2.2.2.7.6 The senior ring assembly is composed of the greater number of heteroatoms of any kind [criterion (f) in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
3,3′-bipyridazine (PIN)
| | 2,3′-bipyridine (PIN) |
| [two heteroatoms per ring is preferred to one heteroatom per ring] |
P-44.2.2.2.7.7 The senior ring assembly is composed of the greater number of heteroatoms in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion(g)in P-44.2.2.2.7].
Example (the symbol > means ‘is senior to’):
 | > |  |
3,3′-bipyrano[3,2-e][1,4]dioxepine (PIN)
| | 2,2′-bi-3,1,5-benzoxadiarsepine (PIN) |
| [three oxygen atoms per ring preferred to one oxygen atom per ring] |
P-44.3 SENIORITY OF ACYCLIC CHAINS (THE PRINCIPAL CHAIN)
In an acyclic compound consisting of individual atoms, alike or different (an acyclic chain), the chain on which the nomenclature and numbering is based is called the ‘principal chain’. When there is a choice for the principal chain, the following criteria are applied, in the order listed, until a decision is reached.
The principal chain:
(a) contains the greater number of heteroatoms of any kind;
(b) has the greater number of skeletal atoms;
(c) contains the greater number of the most senior acyclic heteroatom in the following sequence: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In >Tl.
Each of these criteria is illustrated in P-44.3.1 through P-44.3.3 below.
Further criteria applicable to acyclic chains are found in P-44.4.
P-44.3.1 The principal chain contains the greater number of acyclic heteroatoms of any kind [criterion (a) in P-44.3].
Examples:
| (1) | 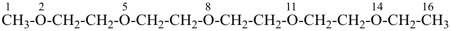 |
| 2,5,8,11,14-pentaoxahexadecane (PIN) |
| is senior to |
| 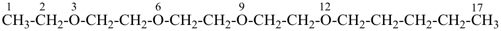 |
| 3,6,9,12-tetraoxaheptadecane (PIN) |
| [five heteroatoms are greater than four] |
| |
| (2) | 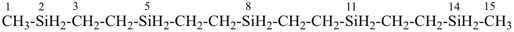 |
| 2,5,8,11,14-pentasilapentadecane (PIN) |
| is senior to |
| 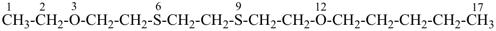 |
| 3,12-dioxa-6,9-dithiaheptadecane (PIN) |
| [five heteroatoms are greater than four] |
P-44.3.2 The principal chain has the greater number of skeletal atoms [criterion (b) in P-44.3].
| In acyclic parent structures the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second criterion. |
|
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| pentane (PIN) | | butane (PIN) |
| [five skeletal atoms is greater than four] |
| |
| (2) | 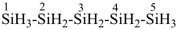 | > |  |
| pentasilane (preselected
name; see P-12.2) | | tetrasilane (preselected
name; see P-12.2) |
| [five skeletal atoms is greater than four)] |
| |
| (3) |  | > |  |
| octane (PIN) | | hept-1-ene (PIN) |
| [eight skeletal atoms is greater than seven] |
| (4) | 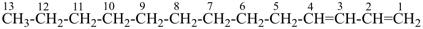 |
| trideca-1,3-diene (PIN) |
| is senior to |
| 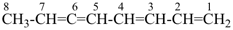 |
| octa-1,3,5,6-tetraene (PIN) |
| [thirteen skeletal atoms is greater than eight] |
| |
| (5) | 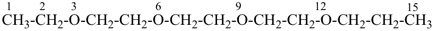 |
| 3,6,9,12-tetraoxapentadecane (PIN) |
| is senior to |
| 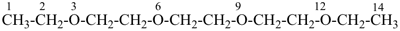 |
| 3,6,9,12-tetraoxatetradecane (PIN) |
| [four heteroatoms in each chain and fifteen skeletal atoms is greater than fourteen] |
| |
| (6) | 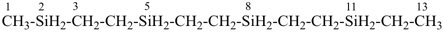 |
| 2,5,8,11-tetrasilatridecane (PIN) |
| is senior to |
|  |
| tetrasilane (preselected name; see P-12.2) |
| [four heteroatoms in each chain and thirteen skeletal atoms is greater than four] |
| |
| (7) |  |
| 2,5,8,11,14-pentasilapentadecane (PIN) |
| is senior to |
| SiH3-O-SiH2-O-SiH3 |
| trisiloxane (preselected name; see P-12.2) |
| [five heteroatoms in each chain; and fifteen skeletal atoms greater than five] |
P-44.3.3 The principal chain contains the greater number of the most senior acyclic heteroatom in the order: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In >Tl [criterion (c) in P-44.3].
Example (the symbol > means ‘is senior to’):
| (1) |  |
| 2,5,8,11-tetraoxatridecane (PIN) |
| is senior to |
| 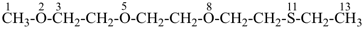 |
| 2,5,8-trioxa-11-thiatridecane (PIN) |
| [four oxygen atoms is more than three] |
| (2) | SiH3-O-SiH3 | > | SiH3-S-SiH3 |
| disiloxane
(preselected name; see P-12.2) | | disilathiane
(preselected name; see P-12.2) |
| [oxygen is senior to sulfur] |
| |
| (3) | SiH3-O-SiH3 | > | SiH3-SiH2-SiH3 |
| disiloxane
(preselected name; see P-12.2) | | trisilane
(preselected name; see P-12.2) |
| [oxygen is senior to silicon] |
P-44.4 SENIORITY CRITERIA APPLICABLE TO RINGS, RING SYSTEMS, OR ACYCLIC CHAINS
P-44.4.1 If the criteria of P-44.1 through P-44.3, where applicable, do not effect a choice of a senior parent structure, the following criteria are applied successively until there are no alternatives remaining. These criteria are illustrated in P-44.4.1.1 through P-44.4.1.12.
The senior ring, ring system, or principal chain:
(a) has the greater number of multiple bonds (P-44.4.1.1);
(b) has the greater number of double bonds (P-44.4.1.2);
(c) has one or more atoms with nonstandard bonding numbers (P-44.4.1.3);
(d) has the lower locants for indicated hydrogen (P-44.4.1.4);
(e) has the lower locants for heteroatoms introduced by skeletal replacement (‘a’) nomenclature as a set (P-44.4.1.5);
(f) has lower locants for heteroatoms introduced by skeletal replacement (‘a’) nomenclature in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl (P-44.4.1.6);
(g) has the lower locant(s) for carbon atoms at fusion sites (P-44.4.1.7);
(h) has the lower locant for an attached group expressed as a suffix (P-44.4.1.8);
(i) has the lower locants for points of attachment (if a substituent group) (P-44.4.1.9);
(j) has the lower locant(s) for endings or prefixes that express changes in the level of hydrogenation, i.e., for ‘ene’ and ‘yne’ endings and ‘hydro/dehydro’ prefixes (P-44.4.1.10);
(k) has one or more isotopically modified atoms (P-44.4.1.11);
(l) has one or more stereogenic centers (P-44.4.1.12).
P-44.4.1.1 The senior ring, ring system or principal chain has the greater number of multiple bonds; for the purpose of this criterion, mancude rings or ring systems are considered as consisting of noncumulative double bonds [criterion (a) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  | > |  |
| benzene (PIN) | | cyclohexene (PIN) | | cyclohexane (PIN) |
| [two double bonds greater than one double bond that is greater than zero] |
| (2) | 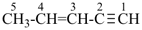 | > | 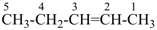 | |
| pent-3-en-1-yne (PIN) | | pent-2-ene (PIN) | |
| [two multiple bonds greater than one] | |
| (3) |  |
| 3,6,9,12-tetraoxatetradec-1-ene (PIN) |
| is senior to |
|  |
| 3,6,9,12-tetraoxatetradecane (PIN) |
| [one double bond greater than zero] |
| |
| (4) |  |
| 1,10(1),4,7(1,4)-tetrabenzenadecaphan-2-en-8-yne (PIN) |
| is senior to |
|  |
| 1,10(1),4,7(1,4)-tetrabenzenadecaphan-2-ene (PIN) |
| [two multiple bonds senior to one multiple bond] |
| or to |
|  |
| 1,10(1),4,7(1,4)-tetrabenzenadecaphan-2-yne (PIN) |
| [one double bond senior to one triple bond] |
P-44.4.1.2 The senior ring, ring system, or principal chain has the greater number of double bonds [criterion (b) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| cycloicosene (PIN) | | cycloicosyne (PIN) |
| [one double bond is senior to one triple bond] |
| |
| (2) |  | > |  |
| cycloicosa-1,8-diene (PIN) | | cycloicos-1-en-3-yne (PIN) |
| [two double bonds are senior to one double bond and one triple bond] |
| |
| (3) |  | > |  |
| 1,4-dihydronaphthalene (PIN) | | 1,2,3,4-tetrahydronaphthalene (PIN) |
| [four double bonds are greater than three] |
| |
| (4) | 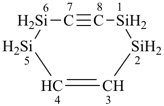 | > | 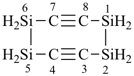 |
| 1,2,5,6-tetrasilacyclooct-3-en-7-yne (PIN) | | 1,2,5,6-tetrasilacycloocta-3,7-diyne (PIN) |
| [one double bond and one triple bond is senior to two triple bonds
since a double bond is senior to a triple bond] |
| |
| (5) |  | > | 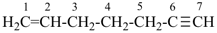 |
| hepta-1,6-diene (PIN) | | hept-1-en-6-yne (PIN) |
| [two double bonds are senior to one double bond and one triple bond
since a double bond is senior to a triple bond] |
| (6) | 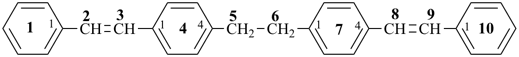 |
| 1,10(1),4,7(1,4)-tetrabenzenadecaphane-2,8-diene (PIN) |
| is senior to |
| 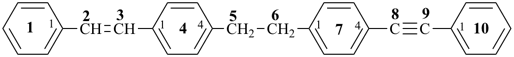 |
| 1,10(1),4,7(1,4)-tetrabenzenadecaphan-2-en-8-yne (PIN) |
| [two double bonds are senior to one double bond and
one triple bond since a double bond is senior to a triple bond] |
P-44.4.1.3 The senior ring, ring system, or principal chain has one or more atoms with nonstandard bonding numbers [criterion (c) in P-44.4.1]. When choices are possible the following criteria are used in turn until a decision is reached.
P-44.4.1.3.1 When a choice is needed between two chains or between two rings or ring systems having skeletal atoms with nonstandard bonding numbers, the one having the maximum number of atoms with nonstandard bonding numbers is chosen as principal chain or senior ring or ring system. If a further choice is needed between the same skeletal atom with different nonstandard bonding numbers, preference for the senior parent structure is given in order of the decreasing numerical value of the bonding number, i.e., λ6 is senior to λ4.
Example (the symbol > means ‘is senior to’):
| (1) | 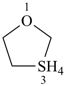 | > | 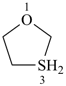 |
| 1,3λ6-oxathiolane (PIN) | | 1,3λ4-oxathiolane (PIN) |
| [the nonstandard bonding number λ6 is senior to λ4] |
| |
| (2) | 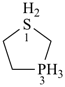 | > | 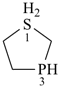 |
| 1λ4,3λ5-thiaphospholane (PIN) | | 1λ4,3-thiaphospholane (PIN) |
| [two nonstandard bonding atoms greater than one] |
| |
| (3) | 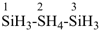 | > | 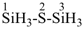 |
| 2λ6-disilathiane
(preselected name; see P-12.2) | | disilathiane
(preselected name; see P-12.2) |
| [the nonstandard bonding number λ6 is senior to λ2] |
| |
| (4) | 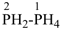 | > |  |
| 1λ5-diphosphane
(preselected name; see P-12.2) | | diphosphane
(preselected name; see P-12.2) |
| [the nonstandard bonding number λ5 is senior to λ3] |
P-44.4.1.3.2 When a choice is needed between two chains or rings or ring systems having skeletal atoms with nonstandard bonding numbers, the one having the lowest locant(s) for (an) atom(s) with nonstandard bonding number(s) is chosen as principal chain or senior ring or ring system. If a further choice is needed, the one with the higher bonding number with the lowest locant is chosen.
Example (the symbol > means ‘is senior to’):
| (1) |  | > | 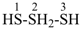 |
| 1λ4-trisulfane
(preselected name, see P-12.2) | | 2λ4-trisulfane
(preselected name, see P-12.2) |
| [the locant ‘1’ for the atom with the nonstandard bonding number is lower than ‘2’] |
| |
| (2) |  | > |  |
| 1λ4,2,3-trithiane (PIN) | | 1,2λ4,3-trithiane (PIN) |
| [the locant ‘1’ for the atom with the nonstandard bonding number is lower than ‘2’] |
| |
| (3) |  | > |  |
| 1λ6,2λ4,3-trithiane (PIN) | | 1λ4,2λ6,3-trithiane (PIN) |
| [the locant sets ‘1,2’ for the atoms with the nonstandard bonding number
are the same,‘1,2’ but the superscript arabic number set giving
the actual nonstandard bonding state is compared
and the number set ‘6,4’ is senior to ‘4,6’] |
P-44.4.1.4 The senior ring or ring system has the lowest locants for indicated hydrogen [criterion (d) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  | |
| 2H-pyran (PIN) | | 4H-pyran (PIN) | |
| [‘2H’ is lower than ‘4H’] | |
| (2) |  |
| 72H-1(4),3,5(2,5)-tripyridina-7(2)-pyranaheptaphane (PIN) |
| is senior to |
| 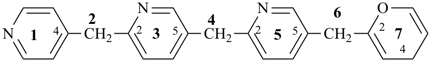 |
| 74H-1(4),3,5(2,5)-tripyridina-7(2)-pyranaheptaphane (PIN) |
| [‘72H’ is lower than ‘74H’] |
P-44.4.1.5 The senior ring, ring system, or principal chain has the lower locants for heteroatoms introduced by skeletal replacement (‘a’) nomenclature as a set [criterion (e) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  |
| 1,7-dioxa-3,5-dithia-4-stannacycloundecane (PIN) |
| is senior to |
|  |
| 1,9-dioxa-4,6-dithia-5-stannacycloundecane (PIN) |
| [the heteroatom locant set ‘1,3,4,5,7’ is lower than ‘1,4,5,6,9’] |
| |
| (2) |  |
| 1,4,6,10-tetraoxa-5λ5-phosphaspiro[4.5]decane (PIN) |
| is senior to |
| 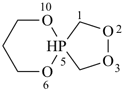 |
| 2,3,6,10-tetraoxa-5λ5-phosphaspiro[4.5]decane (PIN) |
| [the heteroatom locant set ‘1,4,5,6,10’ is lower than ‘2,3,5,6,10’] |
| |
| (3) |  |
| 2-oxa-5-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) |
| is senior to |
|  |
| 2-oxa-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) |
| [the heteroatom locant set ‘2,5’ is lower than ‘2,7’] |
| (4) |  | > | 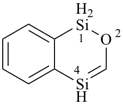 |
| 1H-2,1,3-benzoxadisiline (PIN) | | 1H-2,1,4-benzoxadisiline (PIN) |
| [the heteroatom locant set ‘1,2,3’ is lower than ‘1,2,4’] |
| |
| (5) |  | > |  |
| 3,13-dioxabicyclo[8.2.1]tridecane (PIN) | | 4,13-dioxabicyclo[8.2.1]tridecane (PIN) |
| [the heteroatom locant set ‘3,13’ is lower than ‘4,13’] |
P-44.4.1.6 The senior ring, ring system, or principal chain has lower locants for heteroatoms introduced by skeletal replacement (‘a’) nomenclature in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (f) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| 1,2,3,4,5,7,6,8-hexathiaselenatellurocane
(preselected name, see P-12.2) | | 1,2,3,4,6,7,5,8-hexathiaselenatellurocane
(preselected name, see P-12.2) |
| [the heteroatom locant set ‘1,2,3,4,5,7,6,8’ is lower than ‘1,2,3,4,6,7,5,8’] |
| |
| (2) |  | > |  |
| 1,7,9-trioxa-2-azaspiro[4.5]decane (PIN) | | 2,7,9-trioxa-1-azaspiro[4.5]decane (PIN) |
| [the heteroatom locant set ‘1,7,9,2’ in the order of their citation in the name is lower than ‘2,7,9,1’] |
| |
| (3) |  | > |  |
| 4H,5H-pyrano[4,3-d][1,3,2]dioxathiine (PIN) | | 4H,5H-pyrano[4,3-d][1,2,3]dioxathiine (PIN) |
| [the locant set ‘1,3,6’ for the senior oxygen atoms is lower than ‘2,3,6’] |
| |
| (4) |  | > |  |
| 2-thia-4,6-diazabicyclo[3.2.0]heptane (PIN) | | 4-thia-2,6-diazabicyclo[3.2.0]heptane (PIN) |
| [the locant ‘2’ for the senior sulfur atom is lower than ‘4’] |
| (5) |  | |
| 2-oxa-5-thia-7-selena-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) | |
| is senior to | |
|  | |
| 2-oxa-7-thia-5-selena-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) | |
| [the ‘S’ atom > the ‘Se’ at locant number ‘5’] | |
P-44.4.1.7 The senior fused ring system has the lower locant(s) for carbon atoms at fusion sites [criterion (g) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  | > |  |
| aceanthrylene (PIN) | | acephenanthrylene (PIN) | | fluoranthene (PIN) |
| (I) | | (II) | | (III) |
| [the locant ‘2a’ for the fusion site in (I) is lower than ‘3a’ in (II) and
the locant set ‘3a,5a’ for the fusion sites in (II) is lower than ‘3a,6a’ in (III)] |
| (2) | 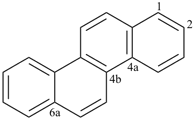 | > |  |
| chrysene (PIN) | | triphenylene (PIN) |
| [the locant set ‘4a,4b,6a’ for the fusion sites is lower than ‘4a,4b,8a’] |
P-44.4.1.8 The senior ring, ring system, or principal chain has the lower locants for characteristic groups cited as a suffix [criterion (h) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| pyridin-2(1H)-one (PIN) | | pyridin-4(1H)-one (PIN) |
| [the locant ‘2’ for the principal characteristic group is lower than ‘4’] |
| (2) | 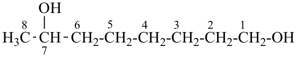 |
| octane-1,7-diol (PIN) |
| is senior to |
| 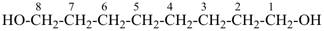 |
| octane-1,8-diol (PIN) |
| [(the locant set ‘1,7’ for the principal characteristic group is lower than ‘1,8’] |
| |
| (3) | 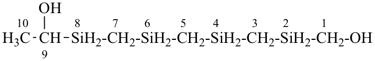 |
| 2,4,6,8-tetrasiladecane-1,9-diol (PIN) |
| is senior to |
| 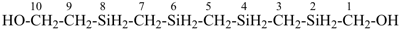 |
| 2,4,6,8-tetrasiladecane-1,10-diol (PIN) |
| [the locant set for the principal characteristic group ‘1,9’ is lower than ‘1,10’] |
| |
| (4) | 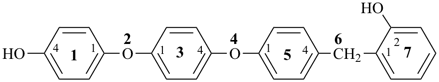 |
| 2,4-dioxa-1,7(1),3,5(1,4)-tetrabenzenaheptaphane-14,72-diol (PIN) |
| is senior to |
| 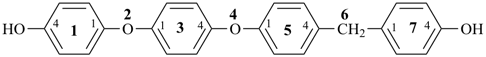 |
| 2,4-dioxa-1,7(1),3,5(1,4)-tetrabenzenaheptaphane-14,74-diol (PIN) |
| [the locant for the principal characteristic group ‘72’ is lower than ‘74’] |
P-44.4.1.9 The senior ring or ring system has the lowest locants for points of attachment (if a substituent group) [criterion (i) in P-44.4.1].
Example (the symbol > means ‘is senior to’):
 | > |  |
| pyridin-2-yl (preferred prefix) | | pyridin-3-yl (preferred prefix) |
| [the locant for the point of attachment ‘2’ is lower than ‘3’] |
P-44.4.1.10 The senior ring, ring system or principal chain has the lowest locants for endings or prefixes that express changes in the degree of hydrogenation, i.e., for ‘ene’ and ‘yne’ endings and ‘hydro/dehydro’ prefixes [criterion (j) in P-44.4.1].
| The prefixes hydro or dehydro are introduced in names by an additive or subtractive operation; thus, they are not included in the category of alphabetizable detachable prefixes describing substitution (P-15.1.3). In names, they occupy a place between nondetachable prefixes and the alphabetizable detachable prefixes. The prefixes hydro or dehydro express modifications of the degree of hydrogenation of a ring or ring system having the maximum number of noncumulative double bonds (a mancude structure) and are treated for numbering like the endings ‘ene’ and ‘yne’, which fulfill the same function. In names, the prefix ‘dehydro’ precedes the prefix ‘hydro’, when both are present. Simple numerical terms, such as ‘di-’,‘tetra-’, etc., are used with ‘hydro’ and ‘dehydro’. |
|
P-44.4.1.10.1 For the endings ‘ene’ and ‘yne’ lower locants are assigned first to the endings as a set without regard to type and then to ‘ene’ endings.
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| cycloicosa-1,3-dien-5-yne (PIN) | | cycloicosa-1,7-dien-3-yne (PIN) |
| [the locant set ‘1,3,5’ for the ‘ene’ and ‘yne’ endings is lower than ‘1,3,7’] |
| |
| (2) |  | > |  |
| cycloicosa-1,3-dien-5-yne (PIN) | | cycloicosa-1,5-dien-3-yne (PIN) |
| [the locant set ‘1,3’ for the ‘ene’ endings is lower than ‘1,5’] |
| (3) | 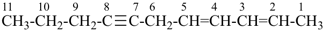 |
| undeca-2,4-dien-7-yne (PIN) |
| is senior to |
| 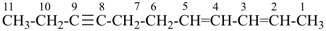 |
| undeca-2,4-dien-8-yne (PIN) |
| [the locant set ‘2,4,7’ for the ‘ene’ and ‘yne’ endings is lower than ‘2,4,8’] |
| |
| (4) | 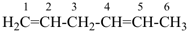 |
| hexa-1,4-diene (PIN) |
| is senior to |
| 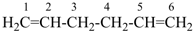 |
| hexa-1,5-diene (PIN) |
| [the locant set ‘1,4’ for the ‘ene’ endings is lower than ‘1,5’] |
| |
| (5) | 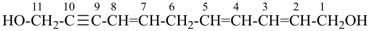 |
| undeca-2,4,7-trien-9-yne-1,11-diol (PIN) |
| is senior to |
| 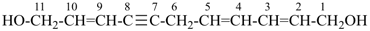 |
| undeca-2,4,9-trien-7-yne-1,11-diol (PIN) |
| [the locant set ‘2,4,7’ for the ‘ene’ endings is lower than ‘2,4,9’] |
| |
| (6) | 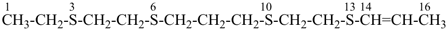 |
| 3,6,10,13-tetrathiahexadec-14-ene (PIN) |
| is senior to |
| 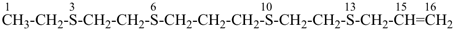 |
| 3,6,10,13-tetrathiahexadec-15-ene (PIN) |
| [the locant ‘14‘ for the ‘ene’ ending is lower than ‘15’] |
| |
| (7) | 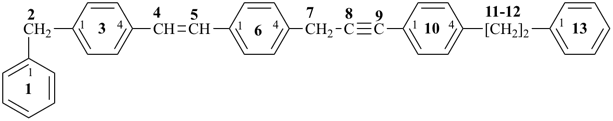 |
| 1,13(1),3,6,10(1,4)-pentabenzenatridecaphane-4-en-8-yne (PIN) |
| is senior to |
|  |
| 1,13(1),3,6,10(1,4)-pentabenzenatridecaphan-4-en-11-yne (PIN) |
| [the locant set ‘4,8’ for the ‘ene’ and ‘yne’ endings is lower than ‘4,11’] |
| |
| (8) |  |
| 1,13(1),3(1,2),6,10(1,4)-pentabenzenatridecaphan-4,8-dien-11-yne (PIN) |
| is senior to |
|  |
| 1,13(1),3(1,2),6,10(1,4)-pentabenzenatridecaphane-4,11-dien-8-yne (PIN) |
| [the locant set ‘4,8’ for the ‘ene’ endings is lower than ‘4,11’] |
P-44.4.1.10.2 For ‘hydro/dehydro’ prefixes, lower locants are assigned as described in P-31.2.
Example (the symbol > means ‘is senior to’):
| (1) |  | > |  |
| 1,2-dihydronaphthalene (PIN) | | 1,4-dihydronaphthalene (PIN) |
| [the locant set for the ‘hydro’ prefixes ‘1,2’ is lower than ‘1,4’] |
| (2) |  |
| 11,12-dihydro-1(2)-quinolina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| is senior to |
|  |
| 11,14-dihydro-1(2)-quinolina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| [the locant set ‘11,12’ for the ‘hydro’ prefixes is lower than ‘11,14’) |
| (3) |  | > |  |
| 1,2-dihydrophosphinine (PIN) | | 1,4-dihydrophosphinine (PIN) |
| [the locant set ‘1,2’ for the ‘hydro’ prefixes is lower than ‘1,4’] |
| |
| (4) |  | > |  |
| 1,2,3,4-tetrahydrophosphinine (PIN) | | 2,3,4,5-tetrahydrophosphinine (PIN) |
| [the locant set ‘1,2,3,4’ for the ‘hydro’ prefixes is lower than ‘2,3,4,5’] |
| |
| (5) |  | > |  |
| 2,3-didehydropyridine (PIN) | | 3,4-didehydropyridine (PIN) |
| [the locant set ‘2,3’ for the ‘dehydro’ prefixes is lower than ‘3,4’] |
Criteria for choosing a preferred IUPAC name based on a senior parent structure as described in P-44 are found in P-45.
P-44.4.1.11 The senior ring, ring system, or principal chain that has one or more isotopically modified atoms [criterion (k) in P-44.4.1].
When there is a choice for the senior parent structure between isotopically modified and isotopically unmodified compounds or between isotopically modified compounds (see Chapter P-8), the senior parent structure is chosen according to the following criteria, applied successively until a decision can be made. In structures and names, nuclide symbols enclosed in parentheses describe isotopic substitution; nuclide symbols enclosed in square brackets describe isotopic labeling (see Chapter P-8).
P-44.4.1.11.1 The senior parent structure contains the greater number of isotopically modified atoms or groups.
Example (the symbol > means ‘is senior to’):
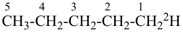 | > | 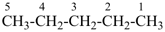 |
| (1-2H1)pentane (PIN) | | pentane (PIN) |
| [one isotopically modified atom is senior to none] |
| |
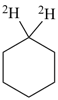 | > | 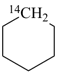 |
| (1,1-2H2)cyclohexane (PIN) | | (14C1)cyclohexane (PIN) |
| [two 2H atoms are senior to one 14C] |
P-44.4.1.11.2 The senior parent structure has the greater number of nuclides of higher atomic number for modified atoms or groups.
Example (the symbol > means ‘is senior to’):
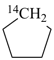 | > | 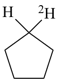 |
| (14C1)cyclopentane (PIN) | | (2H1)cyclopentane (PIN) |
| [14C is senior to 2H] |
| |
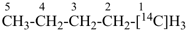 | > | 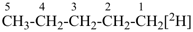 |
| [1-14C]pentane (PIN) | | [1-2H1]pentane (PIN) |
| [14C is senior to 2H] |
P-44.4.1.11.3 The senior parent structure has the greater number of nuclides of higher mass number for modified atoms or groups.
Example (the symbol > means ‘is senior to’):
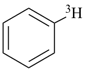 | > | 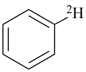 |
| (3H1)benzene (PIN) | | (2H1)benzene (PIN) |
| [3H is senior to 2H] |
| |
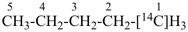 | > | 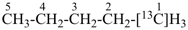 |
| [1-14C]pentane (PIN) | | [1-13C]pentane (PIN) |
| [14C is senior to 13C] |
P-44.4.1.11.4 The senior parent structure has the lowest locant(s) for isotopically modified atoms or groups.
Example (the symbol > means ‘is senior to’):
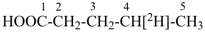 | > | 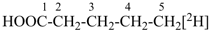 |
| [4-2H1]pentanoic acid (PIN) | | [5-2H1]pentanoic acid (PIN) |
| [4-2H is senior to 5-2H] |
| |
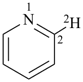 | > | 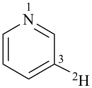 |
| (2-2H)pyridine (PIN) | | (3-2H)pyridine (PIN) |
| [2-2H is senior to 3-2H] |
P-44.4.1.11.5 The senior parent structure has the lower locant(s) for nuclides of higher atomic number for modified atoms or groups;
Example (the symbol > means ‘is senior to’):
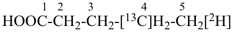 | > | 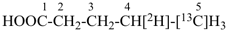 |
| [4-13C,5-2H1]pentanoic acid (PIN) | | [5-13C,4-2H1]pentanoic acid (PIN) |
| [4-13C is senior to 5-13C ] |
P-44.4.1.11.6 The senior parent structure has the lowest locant(s) for nuclides of higher mass number for modified atoms or groups.
Example (the symbol > means ‘is senior to’):
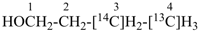 | > | 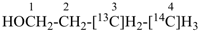 |
| [4-13C,3-14C]butan-1-ol (PIN) | | [3-13C,4-14C]butan-1-ol (PIN) |
| [3-14C is senior to 4-14C] |
P-44.4.1.12 The senior ring, ring system, or principal chain has one or more stereogenic centers [criterion (l) in P-44.4.1].
P-44.4.1.12.1 ‘cis/trans’ Isomerism, the ‘E/Z’ convention
When there is a choice between parent structures that differ only by ‘Z’ and ‘E’ configurations, the senior parent structure contains the greater number of double bonds with ‘Z’ configuration; when a further choice is required, the senior parent structure has the lowest locant set for the double bond(s) with the ‘Z’ configuration. For the meaning of ‘Z’ and ‘E’ see Chapter P-9.
Example (the symbol > means ‘is senior to’):
| (1) | 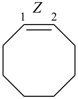 | > | 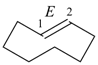 |
| (Z)-cyclooctene (PIN)
cis-cyclooctene | | (E)-cyclooctene (PIN)
trans-cyclooctene |
| [‘Z’ is senior to ‘E’] |
| |
| (2) |  | > |  |
| (2Z)-2-methylbut-2-enoic acid (PIN) | | (2E)-2-methylbut-2-enoic acid (PIN) |
| [‘Z’ is senior to ‘E’] |
| |
| (3) |  | > |  |
| (4Z)-hex-4-enenitrile (PIN) | | (4E)-hex-4-enenitrile (PIN) |
| [‘Z’ is senior to ‘E’] |
| (4) | 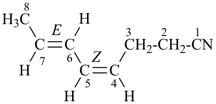 |
| (4Z,6E)-octa-4,6-dienoic acid (PIN) |
| is senior to |
| 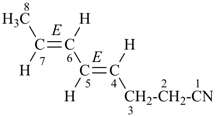 |
| (4E,6E)-octa-4,6-dienoic acid (PIN) |
| [‘Z,E’ is senior to ‘E,E’] |
| |
| (5) |  |
| (4Z,7E)-nona-4,7-dienoic acid (PIN) |
| is senior to |
|  |
| (4E,7Z)-nona-4,7-dienoic acid (PIN) |
| [‘4Z,7E’ is senior to ‘4E,7Z’] |
P-44.4.1.12.2 Enantiomerism, the ‘R’ and ‘S’ stereodescriptors
When there is a choice between parent structures differing only by the configurations of the chirality centers, the principal chain or the senior ring system is chosen by applying the CIP sequence rules 4 and 5, in the order: like stereodescriptors such as ‘RR’, ‘SS’ have priority over unlike ‘RS’ and ‘SR’ (‘l’ has priority over ‘u’), the ‘r’ over ‘s’, then ‘R’ over ‘S’. The CIP Sequence Rules are described in Chapter P-9.
Example (the symbol > means ‘is senior to’):
| (1) | 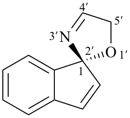 |
| (1R)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN) |
| is senior to |
| 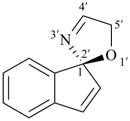 |
| (1S)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN) |
| [‘R’ is senior to ‘S’] |
| |
| (2) | 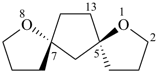 |
| (5R,7R)-1,8-dioxadispiro[4.1.47.25]tridecane (PIN) |
| is senior to |
| 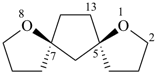 |
| (5R,7S)-1,8-dioxadispiro[4.1.47.25]tridecane (PIN) |
| [‘RR’ is senior to ‘RS’] |
P-44.4.1.12.3 ‘cis/trans’ Isomerism (the ‘E/Z’ convention) and enantiomerism (the ‘R/S’ convention)
When there is a choice between parent structures that contain both ‘E/Z’ (or cis/trans) and ‘R/S’ descriptors the ‘E/Z’ (or cis/trans) descriptors are preferred to the ‘R/S’ descriptors.
Example
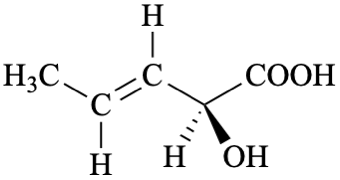 | > | 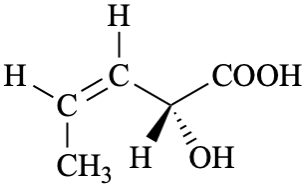 |
| (2R,3Z)-2-hydroxypent-3-enoic acid | | (2S,3E)-2-hydroxypent-3-enoic acid |
[Z is senior to E] 
P-45 SELECTION OF PREFERRED IUPAC NAMES
P-45.0 INTRODUCTION
Two or more names may result based on the same senior parent structure selected according to P-44, because of different substitution patterns or multiple occurrences of the same senior parent structure. A parent structure is defined (P-15.1) as a parent hydride, for example, benzene, a functionalized parent hydride, for example cyclohexanol, or a functional parent compound, for example, acetic acid. Application of the criteria in this section will generate the preferred IUPAC name.
P-45.1 Multiplication of identical senior parent structures
P-45.2 Criteria related to number and location of substituent groups
P-45.3 Criteria related only to substituents with nonstandard bonding numbers, other criteria being equal
P-45.4 Criteria related only to isotopic modification of substituents
P-45.5 Criteria related to alphanumerical order of names
P-45.6 Criteria related only to configuration
P-45.1 MULTIPLICATION OF IDENTICAL SENIOR PARENT STRUCTURES
P-45.1.1 Multiplicative nomenclature is senior to substitutive nomenclature for generating preferred IUPAC names to express multiple occurrences of identical senior parent structures, other than alkanes, in the name of the parent structure (see P-51.3.1). In most cases multiplicative names are shorter than regular substitutive names. A preferred IUPAC name is generated by multiplicative nomenclature when the following criteria for its use are met (see P-51.3.1).
(1) the linking bonds (single or multiple) between the central substituent group of the multiplicative group and all subsequent structural units are identical; and
(2) the multiplicative groups, other than the central multiplicative group, are symmetrically substituted; and
(3) the locants of all substituent groups on the identical parent structures, including suffix groups, are identical.
When these conditions are not met, substitutive nomenclature generates preferred IUPAC names.
Example:
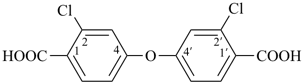
4,4′-oxybis(2-chlorobenzoic acid) (PIN, multiplicative name)
but
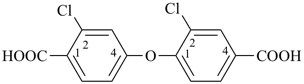
4-(4-carboxy-2-chlorophenoxy)-2-chlorobenzoic acid (PIN, substitutive name)
P-45.1.2 When two or more parent structures, rings, ring systems, or chains, satisfy the requirements for multiplicative nomenclature (see P-15.3), the structure chosen as the parent structure to be multiplied is the more numerous.
Examples:
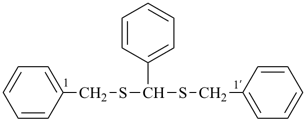
1,1′-[(phenylmethylene)bis(sulfanediylmethylene)]dibenzene (PIN)
(multiplication of three benzene rings is not possible)
2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
4,4′,4′′-(ethene-1,1,2-triyl)trianiline (PIN)
[not 4,4′-[2-(4-aminophenyl)ethene-1,1-diyl]dianiline;
the PIN multiplies three parent structures;
the second name multiplies only two]
[phosphanetriyltris(methylene)]tris(phosphonic acid) (PIN)
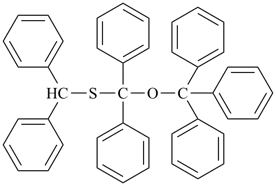
1,1′,1′′-({[(diphenylmethyl)sulfanyl]diphenylmethoxy}methanetriyl)tribenzene (PIN)
(not 1,1′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methylene)dibenzene;
the PIN multiplies three parent structures;
the second name multiplies only two)
P-45.2 CRITERIA RELATED TO NUMBER AND LOCATION OF SUBSTITUENT GROUPS
The following criteria are applied, in turn, until a decision is reached. The preferred IUPAC name is the name based on the senior parent structure that has:
P-45.2.1 the maximum number of substituent groups cited as prefixes;
P-45.2.2 the lower locant set for substituent groups cited as prefixes;
P-45.2.3 the lower locant set for substituent groups in order of citation in the name
P-45.2.1 The preferred IUPAC name is based on the senior parent structure that has the maximum number of substituents cited as prefixes (other than ‘hydro/dehydro’) to the parent structure.
Examples:
| (1) |  |
| 4-methoxy-N-phenylaniline (PIN)
[not N-(4-methoxyphenyl)aniline;
the PIN parent structure has more substituents,
two simple substituents vs. one compound substituent] |
| |
| (2) |  |
| 4-chloro-2-[(3-cyanophenyl)methyl]benzonitrile (PIN)
[not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile;
the PIN parent structure has more substituents,
two (one simple and one complex) substituents vs. one complex substituent] |
| |
| (3) |  |
| 1-methyl-4-(phenoxymethyl)benzene (PIN)
[not [(4-methylphenyl)methoxy]benzene;
the PIN parent structure has more substituents;
two (one simple and one compound) substituent vs. one complex substituent] |
| |
| (4) | 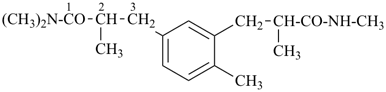 |
| N,N,2-trimethyl-3-{4-methyl-3-[2-methyl-3-(methylamino)-3-oxopropyl]phenyl}propanamide (PIN)
[not 3-{5-[(3-dimethylamino)-2-methyl-3-oxopropyl]-2-methylphenyl}-N,2-dimethylpropanamide;
the PIN parent structure has more substituents,
four (three simple and one complex) substituents vs. three (two simple and one complex)] |
| |
| (5) | 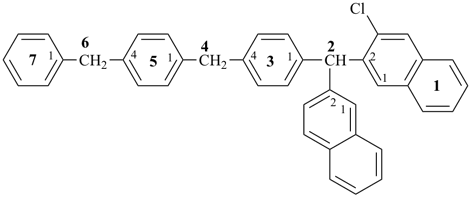 |
| 13-chloro-2-(naphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
[not 2-(3-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane;
the PIN parent structure has more substituents, two simple substituents vs. one compound substituent] |
| |
| (6) | 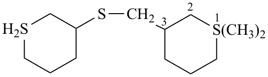 |
| 1,1-dimethyl-3-{[(1λ4-thian-3-yl)sulfanyl]methyl}-1λ4-thiane (PIN)
[not 3-{[(1,1-dimethyl-1λ4-thian-3-yl)methyl]sulfanyl}-1λ4-thiane;
the PIN parent structure has more substituents,
three (two simple and one complex) substituents vs. one complex substituent] |
| |
| (7) | 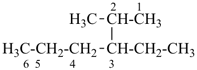 |
| 3-ethyl-2-methylhexane (PIN)
[not 3-isopropylhexane or 3-(propan-2-yl)hexane;
the PIN parent structure has more substituents,
two simple substituents vs. one simple substituent) |
| |
| (8) | 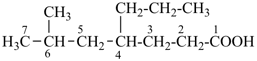 |
| 6-methyl-4-propylheptanoic acid (PIN)
[not 4-(2-methylpropyl)heptanoic acid;
the principal chain has more substituent,
two simple substituents vs. one compound substituent] |
| |
| (9) | 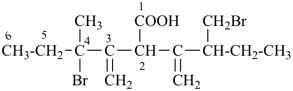 |
| 4-bromo-2-[3-(bromomethyl)pent-1-en-2-yl]-4-methyl-3-methylidenehexanoic acid (PIN)
[not 4-(bromomethyl)-2-(3-bromo-3-methylpent-1-en-2-yl)-3-methylidenehexanoic
acid;
the PIN parent chain has more substituents;
four (three simple and one complex) substituents vs. three (one simple and two compound)]
4-bromo-2-[2-(bromomethyl)-1-methylidenebutyl]-4-methyl-3-methylidenehexanoic acid |
| |
| (10) | 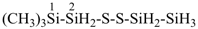 |
| 2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
[not [2-(2,2,2-trimethyldisilanyl)disulfan-1-yl]disilane;
the PIN parent structure has more substituents,
four (three simple and one compound) substituents vs. one complex substituent] |
| |
| (11) | 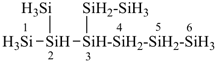 |
| 3-disilanyl-2-silylhexasilane (preselected name, see P-12.2)
[not 3-(trisilan-2-yl)hexasilane or 3-(1-silyldisilanyl)hexasilane;
the PIN parent chain has more substituents, two simple substituents vs. one simple or one compound substituent] |
| |
| (12) | 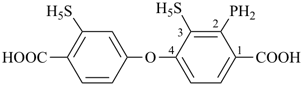 |
| 4-[4-carboxy-3-(λ6-sulfanyl)phenoxy]-2-phosphanyl-3-(λ6-sulfanyl)benzoic acid (PIN)
[not 4-[4-carboxy-3-phosphanyl-2-(λ6-sulfanyl)phenoxy]-2-(λ6-sulfanyl)benzoic acid)
the PIN parent structure has more substituents,
three (two simple and one compound) substituents vs. two (one simple and one complex)] |
| |
| (13) | 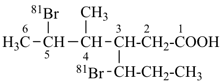 |
| 5-(81Br)bromo-3-[1-(81Br)bromopropyl]-4-methylhexanoic acid (PIN)
[not 4-(81Br)bromo-3-[3-(81Br)bromobutan-2-yl]hexanoic acid;
the PIN parent chain has more substituents,
three (two simple and one compound) vs. two (one simple and one compound)] |
P-45.2.2 The preferred IUPAC name is based on the senior parent structure that has the lower locant or set of locants for substituents cited as prefixes (other than ‘hydro/dehydro’) to the parent structure.
Examples:
| (1) |  |
| 2-(2-amino-4-methylphenoxy)-N-methylaniline (PIN)
[not 5-methyl-2-[2-(methylamino)phenoxy]aniline;
the locant set ‘N,2’ in the PIN is lower than ‘2,5’] |
| |
| (2) |  |
| 1-bromo-3-chloro-6-nitro-2-[2-(1,3,7-trifluoronaphthalen-2- yl)ethyl]naphthalene (PIN)
[not 2-[2-(1-bromo-3-chloro-6-nitronaphthalen-2-yl)ethyl]-1,3,7-trifluoronaphthalene;
the locant set ‘1,2,3,6’ in the PIN is lower than ‘1,2,3,7’] |
| |
| (3) | 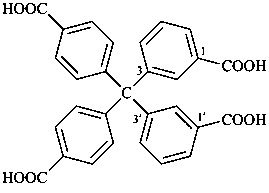 |
| 3,3′-[bis(4-carboxyphenyl)methylene]dibenzoic acid (PIN)
[not 4,4′-[bis(3-carboxyphenyl)methylene]dibenzoic acid;
the locant set 3,3′ in the PIN is lower than 4,4′] |
| |
| (4) | 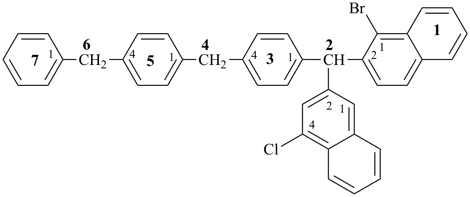 |
| 11-bromo-2-(4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
[not 2-(1-bromonaphthalen-2-yl)-14-chloro-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane;
the locant set ‘11,2’ in the PIN is lower than the locant set ‘14,2’] |
| |
| (5) |  |
| 3-[5-(3-amino-2-methyl-3-oxopropyl)-2-methylphenyl]-N-methylpropanamide (PIN)
[not 2-methyl-3-{4-methyl-3-[3-(methylamino)-3-oxopropyl]phenyl}propanamide;
the locant set ‘N,3’ in the PIN is lower than ‘2,3’] |
| |
| (6) |  |
| 5,6-dibromo-4-(1-chloro-3-nitropropyl)heptanoic acid (PIN)
[not 5-chloro-4-(1,2-dibromopropyl)-7-nitroheptanoic acid;
the locant set ‘4,5,6’ in the PIN is lower than ‘4,5,7’] |
| |
| (7) |  |
| 2-amino-5-(2-chloro-4-hydroxybutyl)-6-methylnonane-1,9-diol (PIN)
[not 2-amino-7-chloro-5-(5-hydroxypentan-2-yl)nonane-1,9-diol;
not
5-(3-amino-4-hydroxybutyl)-3-chloro-6-methylnonane-1,9-diol;
the locant set ‘2,5,6’ in the PIN is lower than the locant set ‘2,5,7’ or ‘3,5,6’] |
| |
| (8) | 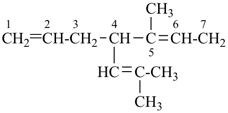 |
| 5-methyl-4-(2-methylprop-1-en-1-yl)hepta-1,5-diene (PIN)
[not 4-(but-2-en-2-yl)-6-methylhepta-1,5-diene;
the locant set ‘4,5’ in the PIN is lower than ‘4,6’] |
| |
| (9) | 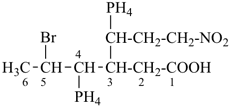 |
| 5-bromo-3-[3-nitro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid (PIN)
[not 3-[2-bromo-1-(λ5-phosphanyl)propyl]-6-nitro-4-(λ5-phosphanyl)hexanoic acid,
the locant set ‘3,4,5’ in the PIN is lower than the locant set ‘3,4,6’] |
| |
| (10) | 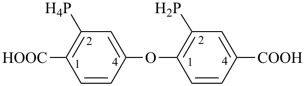 |
| 4-(4-carboxy-2-phosphanylphenoxy)-2-(λ5-phosphanyl)benzoic acid (PIN)
[not 4-[4-carboxy-3-(λ5-phosphanyl)phenoxy]-3-phosphanylbenzoic acid;
the locant set ‘2,4’ in the PIN is lower than ‘3,4’] |
| |
| (11) | 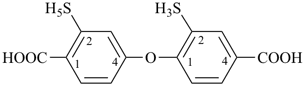 |
| 4-[4-carboxy-2-(λ4-sulfanyl)phenoxy]-2-(λ6-sulfanyl)benzoic acid (PIN)
[not 4-[4-carboxy-3-(λ6-sulfanyl)phenoxy]-3-(λ4-sulfanyl)benzoic acid;
the locant set ‘2,4’ in the PIN is lower than ‘3,4’] |
| |
| (12) | 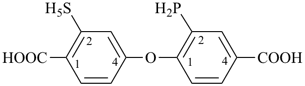 |
| 4-(4-carboxy-2-phosphanylphenoxy)-2-(λ6-sulfanyl)benzoic acid (PIN)
[not 4-[4-carboxy-3-(λ6-sulfanyl)phenoxy]-3-phosphanylbenzoic acid;
the locant set ‘2,4’ in the PIN is lower than ‘3,4’] |
| |
| (13) |  |
| 3-{[3-(2H2)amino-5-methylcyclohexa-1,5-dien-1-yl]sulfanyl}-2- methylcyclohexa-2,4-dien-1-(2H2)amine (PIN)
[not 3-{[3-(2H2)amino-2-methylcyclohexa-1,5-dien-1-yl]sulfanyl}-5-methylcyclohexa-2,4-dien-1-(2H2)amine,
the locant set ‘2,3’ in the PIN is lower than ‘3,5’] |
| |
| (14) |  |
| 4-(81Br)bromo-3-[2-(81Br)bromopropyl]hexanoic acid (PIN)
[not 5-(81Br)bromo-3-[1-(81Br)bromopropyl]hexanoic acid,
the locant set in the PIN, ‘3,4’ is lower than ‘3,5’] |
| |
| (15) |  |
| 3-chloro-5-(3-hydroxybutyl)-4,6-dimethylnonane-2,8-diol (PIN)
[not 7-chloro-5-(3-hydroxybutyl)-4,6-dimethylnonane-2,8-diol:
the locant set of the PIN is ‘3,4,5,6’ which is lower than ‘4,5,6,7’] |
| |
P-45.2.3 The preferred IUPAC name is based on the senior parent structure that has the lower locant or set of locants for substituents cited as prefixes to the parent structure (other than ‘hydro/dehydro’ prefixes) in their order of citation in the name.
Examples:
| (1) |  |
| 3-chloro-7-[(4-chloro-3-nitroquinolin-7-yl)sulfanyl]-4-nitroquinoline (PIN)
[not 4-chloro-7-[(3-chloro-4-nitroquinolin-7-yl)sulfanyl]-3-nitroquinoline,
the locant sets are the same in both names, i.e., ‘3,4,7’, but in their order
of appearance in the name the locant set ‘3,7,4’ in the PIN is lower than ‘4,7,3’] |
| |
| (2) |  |
| 2-bromo-N-(4-bromo-2-chlorophenyl)-4-chloroaniline (PIN)
[not 4-bromo-N-(2-bromo-4-chlorophenyl)-2-chloroaniline;
the locant sets are the same, i.e., ‘N,2,4’, but in their order
of appearance in the name the locant set ‘2,N,4’ in the PIN is lower than ‘4,N,2’] |
| |
| (3) |  |
| 1-ethyl-7-[(7-ethyl-8-propylnaphthalen-2-yl)oxy]-2-propylnaphthalene (PIN)
[not 2-ethyl-7-[(8-ethyl-7-propylnaphthalen-2-yl)oxy]-1-propylnaphthalene,
the locant sets are the same in both names, i.e., ‘1,2,7’, but in their order
of appearance in the name, the locant set in the PIN ‘1,7,2’ is lower than ‘2,7,1’] |
| |
| (4) |  |
| 5-bromo-4-(2-bromo-1-fluoropropyl)-6-fluoroheptanoic acid (PIN)
[not 6-bromo-4-(1-bromo-2-fluoropropyl)-5-fluoroheptanoic acid;
the locant sets are the same in both names, i.e., ‘4,5,6’, but in their order
of appearance in the name the locant set ‘5,4,6’ in the PIN is lower than ‘6,4,5’] |
| |
| (5) | 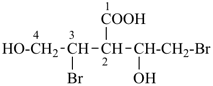 |
| 3-bromo-2-(2-bromo-1-hydroxyethyl)-4-hydroxybutanoic acid (PIN)
[not 4-bromo-2-(1-bromo-2-hydroxyethyl)-3-hydroxybutanoic acid;
the locant sets are the same in both names, i.e., ‘2,3,4’, but in their order
of appearance in the name, the locant set ‘3,2,4’ in the PIN is lower than ‘4,2,3’] |
| |
| (6) | 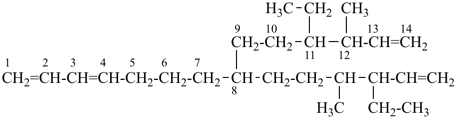
(I) |
| 11-ethyl-8-(4-ethyl-3-methylhex-5-en-1-yl)-12-methyltetradeca-1,3,13-triene (PIN) (I)
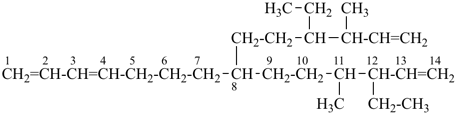
(II)
[not 12-ethyl-8-(3-ethyl-4-methylhex-5-en-1-yl)-11-methyltetradeca-1,3,13-triene (II)
the locant sets are the same in both names, i.e., ‘8,11,12’, but in their order
of appearance in the name the locant set ‘11,8,12’ in the PIN is lower than ‘12,8,11’] |
| |
| (7) | 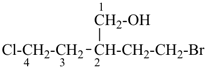 |
| 2-(2-bromoethyl)-4-chlorobutan-1-ol (PIN)
[not 4-bromo-2-(2-chloroethyl)butan-1-ol
the locant sets are the same in both names, i.e., ‘2,4’ but in the order
of appearance in the name, the locant set ‘2,4’ in the PIN is lower than ‘4,2’] |
| |
| (8) | 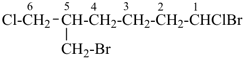 |
| 1-bromo-5-(bromomethyl)-1,6-dichlorohexane (PIN)
[not 1,6-dibromo-1-chloro-5-(chloromethyl)hexane;
the locant sets are the same in both names, i.e., ‘1,1,5,6’ but in their order
of appearance in the name, the locant set ‘1,5,1,6’ is lower than ‘1,6,1,5’] |
| |
| (9) | 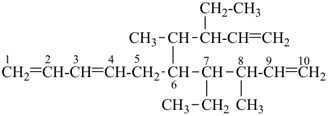 |
| 7-ethyl-6-(3-ethylpent-4-en-2-yl)-8-methyldeca-1,3,9-triene (PIN)
not
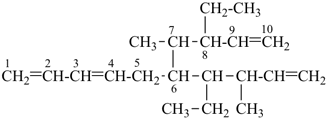
8-ethyl-7-methyl-6-(4-methylhex-5-en-3-yl)deca-1,3,9-triene
[the locant sets are the same in both names, i.e. ‘6,7,8’ but in their order
of appearance in the name, the locant set ‘7,6,8’ is lower than ‘8,7,6’] |
| |
| (10) | 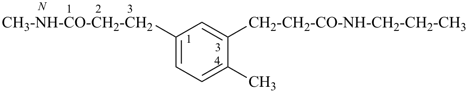 |
| N-methyl-3-{4-methyl-3-[3-oxo-3-(propylamino)propyl]phenyl}propanamide (PIN)
[not 3-{2-methyl-5-[3-(methylamino)-3-oxopropyl]phenyl}-N-propylpropanamide;
the locant sets are the same in both names, i.e. ‘N,3’ but in their order
of appearance in the name, the locant set ‘N,3’ in the PIN is lower than ‘3,N’] |
| |
| (11) | 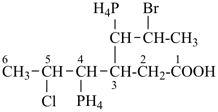 |
| 3-[2-bromo-1-(λ5-phosphanyl)propyl]-5-chloro-4-(λ5-phosphanyl)hexanoic acid (PIN)
[not 5-bromo-3-[2-chloro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid;
the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order
of appearance in the name, the locant set ‘3,5,4’ in the PIN is lower than ‘5,3,4’] |
| |
| (12) | 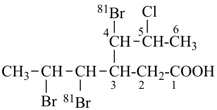 |
| 4-(81Br)bromo-3-[1-(81Br)bromo-2-bromopropyl]-5-chlorohexanoic acid (PIN)
[not 4-(81Br)bromo-5-bromo-3-[1-(81Br)bromo-2-chloropropyl]hexanoic acid;
the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order
of appearance in the name, the locant set ‘4,3,5’ in the PIN is lower than ‘4,5,3’] |
| |
| (13) | 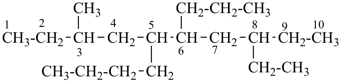 |
| 5-butyl-8-ethyl-3-methyl-6-propyldecane (PIN)
[not 6-butyl-3-ethyl-8-methyl-5-propyldecane;
both have the set of locants ‘3,5,6,8’ but the PIN has them in the order ‘5,8,3,6’ which is lower than ‘6,3,8,5’] |
| |
| (14) | 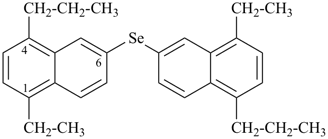 |
| 1-ethyl-6-[(8-ethyl-5-propylnaphthalen-2-yl)selanyl]-4-propylnaphthalene (PIN)
[not 4-ethyl-6-[(5-ethyl-8-propylnaphthalen-2-yl)selanyl]-1-propylnaphthalene:
both have the locant set ‘1,4,6’ but the PIN set is in the order ‘1,6,4’ which is lower than ‘4,6,1’] |
| |
| (15) | 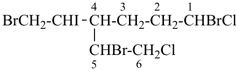 |
| 1,5-dibromo-4-(2-bromo-1-iodoethyl)-1,6-dichlorohexane (PIN)
[not 1,6-dibromo-4-(1-bromo-2-chloroethyl)-1-chloro-5-iodohexane;
both have the locant set ‘1,1,4,5,6’ but the PIN set is in the order ‘1,5,4,1,6’ which is lower than ‘1,6,4,1,5’] |
| |
P-45.3 CRITERIA RELATED ONLY TO SUBSTITUENTS WITH NONSTANDARD BONDING NUMBERS, OTHER CRITERIA BEING EQUAL
The following criteria are relative only to substituents with nonstandard bonding numbers applied in turn, until a decision is reached, earlier criteria having been satisfied.
P-45.3.1 the maximum number of substituent group(s) with the higher bonding number cited as prefixes;
P-45.3.2 the lower locant set for substituent group(s) with the higher bonding number cited as prefixes.
P-45.3.1 The preferred IUPAC name is based on the senior parent structure that has the maximum number of substituent group(s) with the higher bonding number cited as prefixes and directly connected to the parent structure.
Examples:
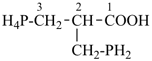
3-(λ5-phosphanyl)-2-(phosphanylmethyl)propanoic acid (PIN)
[not 3-phosphanyl-2-(λ5-phosphanylmethyl)propanoic acid;
the PIN parent structure contains the substituent group having the highest bonding number, ‘λ5’ > ‘λ3’]
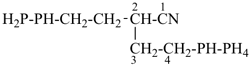
4-(2λ5-diphosphan-1-yl)-2-(2-diphosphanylethyl)butanenitrile (PIN)
[not 4-diphosphanyl-2-[2-(2λ5-diphosphan-1-yl)ethyl]butanenitrile;
the PIN parent structure contains the substituent group having the highest bonding number, ‘λ5’ > ‘λ3’]
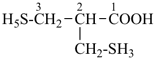
3-(λ6-sulfanyl)-2-(λ4-sulfanylmethyl)propanoic acid (PIN)
[not 3-(λ4-sulfanyl)-2-(λ6-sulfanylmethyl)propanoic acid;
the PIN parent structure contains the substituent group having the highest bonding number, ‘λ6’ > ‘λ4’]
P-45.3.2 The preferred IUPAC name has the lower locant set for substituent group(s) with the higher bonding number(s) cited as prefixes.
Example:
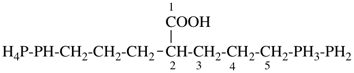
5-(1λ5-diphospan-1-yl)-2-[3-(2λ5-diphospan-1-yl)propyl]pentanoic acid (PIN)
[not 5-(2λ5-diphospan-1-yl)-2-[3-(1λ5-diphospan-1-yl)propyl]pentanoic acid]
The locant ‘1λ5’ for the nonstandard bonding number directly bonded to the parent structure is lower than ‘2λ5’.
P-45.4 CRITERIA RELATED ONLY TO ISOTOPIC MODIFICATION OF SUBSTITUENTS
The following criteria are relative only to substituents with isotopic modification applied in turn, until a decision is reached, earlier criteria having been satisfied.
P-45.4.1 The preferred IUPAC name is based on the senior parent structure that has the lowest locant(s) for isotopically modified substituent groups.
Example:
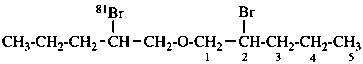
2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane (PIN)
[not 2-(81Br)bromo-1-[(2-bromopentyl)oxy]pentane]
The isotopically modified substituent for the PIN is attached at ‘1’ which is lower than ‘2’.
P-45.4.2 The preferred IUPAC name is based on the senior parent structure that has the lowest locant(s) for nuclides of higher atomic number.
Example:
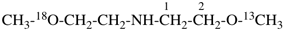
2-(13C)methoxy-N-[2-(18O)methoxyethyl]ethan-1-amine (PIN)
[not 2-(18O)methoxy-N-[2-(13C)methoxyethyl]ethan-1-amine]
18O > 13C; the 18O isotopically modified substituent for the PIN is attached at ‘N’ which is lower than ‘2’.  P-45.4.3 The preferred IUPAC name is based on the senior parent structure that has the lowest locant(s) for nuclides of higher mass number.
P-45.4.3 The preferred IUPAC name is based on the senior parent structure that has the lowest locant(s) for nuclides of higher mass number.
Example:
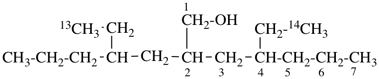
4-[2-13C]ethyl-2-(2-[2-14C]ethylpentyl)heptan-1-ol (PIN)
[not 4-[2-14C]ethyl-2-(2-[2-13C]ethylpentyl)heptan-1-ol
14C > 13C; the 14C isotopically modified substituent for the PIN is attached at ‘2’ which is lower than ‘4’  P-45.5 CRITERIA RELATED TO ALPHANUMERICAL ORDER OF NAMES
P-45.5 CRITERIA RELATED TO ALPHANUMERICAL ORDER OF NAMES
The preferred IUPAC name is the name that is earlier in alphanumerical order (see P-14.5). Alphabetic letters are considered first in the order that they appear in the name; all Roman letters are considered before any italic letters, unless the latter are used as locants or are a part of a compound or composite locant, for example, ‘N’ and ‘4a’. Then, if still there is a choice, numerical locants are considered in the order of their appearance in the name.
Examples:
| (1) | 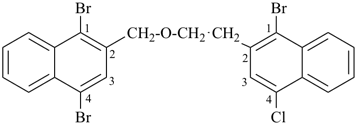 |
| 1-bromo-4-chloro-2-{2-[(1,4-dibromonaphthalen-2-yl)methoxy]ethyl}naphthalene (PIN)
[not 1,4-dibromo-2-{[2-(1-bromo-4-chloronaphthalen-2-yl)ethoxy]methyl}naphthalene;
in both names the locant set is ‘1,2,4’, and the locants appear in the name in the same order ‘1,4,2’ so
no decision can be made by P-45.2.2 or P-45.2.3; but ‘bromo’ in the PIN is earlier alphabetically than ‘dibromo’.] |
| |
| (2) | 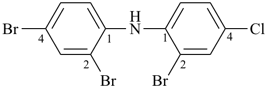 |
| 2-bromo-4-chloro-N-(2,4-dibromophenyl)aniline (PIN)
[not 2,4-dibromo-N-(2-bromo-4-chlorophenyl)aniline;
‘bromo’. in the PIN is earlier alphabetically than ‘dibromo’.] |
| |
| (3) | 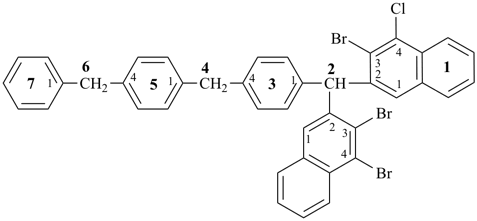 |
| 13-bromo-14-chloro-2-(3,4-dibromonaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
[not 13,14-dibromo-2-(3-bromo-4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane;
‘bromo’ in the PIN is earlier alphabetically than ‘dibromo’.] |
| |
| (4) | 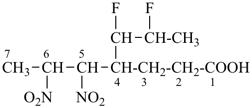 |
| 4-(1,2-difluoropropyl)-5,6-dinitroheptanoic acid (PIN)
[not 4-(1,2-dinitropropyl)-5,6-difluoroheptanoic acid;
‘difluoro’ in the PIN is earlier alphabetically than ‘dinitro’] |
| |
| (5) | 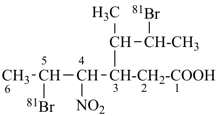 |
| 5-(81Br)bromo-3-[3-(81Br)bromobutan-2-yl]-4-nitrohexanoic acid (PIN)
[not 5-(81Br)bromo-3-[2-(81Br)bromo-1-nitropropyl]-4-methylhexanoic acid;
‘bromo-bromo-butanyl’ is lower alphanumerically than ‘bromo-bromo-nitro’]
[Note: The ‘B’ of the element symbol ‘Br’ is not a factor in the alphabetization] |
P-45.6 CRITERIA RELATED ONLY TO CONFIGURATION
P-45.6.1 Introduction
This section is concerned only with the main principles for specification of configuration in names of organic compounds. The spatial structure of an organic compound is systematically indicated by one or more affixes added to a name that does not itself prescribe configuration; such affixes are generally called ‘stereodescriptors’ and do not change the preferred IUPAC name of a compound established by the principles of nomenclature described in other sections of these recommendations (see P-44 and P-45), under the condition that no choice has yet been encountered during the construction of the preferred IUPAC name. When there is a choice, the preferred IUPAC name must be constructed in conformity with the rules for choosing the principal chain or the senior ring or ring system and also with the priority of the stereodescriptors. Thus, stereoisomers, such as enantiomers and cis/trans isomers, have names, substitutive or multiplicative, that differ only in the stereodescriptors used. Stereodescriptors describing preferred IUPAC names are all Cahn-Ingold-Prelog CIP descriptors, such as E, Z, R, S, r and s discussed and illustrated in Chapter P-9.
P-45.6.2 General principles. The choice of a parent structure based on the number and location of multiple bonds and double bonds (see P-44.4.1.1, P-44.4.1.2, and P-44.4.1.12) is not configuration dependent. As an example, the configuration of the double bond may be Z or E in the following alkene that is named according to P-31.1. The names with and without stereodescriptors are the same, i.e. butene. Preferred IUPAC names are generated from the preferred IUPAC name of the alkene when stereodescriptors are correct.
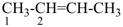
but-2-ene (PIN)
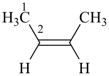
(2Z)-but-2-ene (PIN)
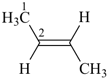
(2E)-but-2-ene (PIN)
In contrast, multiplicative names (see P-45.1 and P-15.3) are configuration dependent as shown below. Two different procedures are required: a multiplicative name to describe identical configurations (‘E’ or ‘Z’; or ‘R’ or ‘S’) in identical units and a substitutive name to describe different configurations (‘E’ and ‘Z’ or ‘R’ and ‘S’) as shown by the first example below.
Example 1:
(1E,1′E)-1,1′-sulfanediyldi(cyclooct-1-ene) (PIN)
(a multiplicative name)
(1Z,3S)-3-{2-[(1R,2E)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene (PIN)
(1-cis,3S)-3-{2-[(1R,2-trans)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene
(substitutive names; for the priority of ‘Z’ > ‘E’ > ‘R’ > ‘S’, see P-92)
Example 2:
1,1′-sulfanediylbis{4-[(2R)-butan-2-yl]benzene} (PIN)
(a multiplicative name)
1-[(2R)-butan-2-yl]-4-({4-[(2S)-butan-2-yl]phenyl}sulfanyl)benzene (PIN)
(a substitutive name; a multiplicative name is not allowed)
[not 1-[(2S)-butan-2-yl]-4-({4-[(2R)-butan-2-yl]phenyl}sulfanyl)benzene;
also a substitutive name but since the alphabetic characters and locants
(ignoring the configuration symbols)
are identical the configurational symbols are compared and ‘R’ precedes ‘S’]
Example 3:
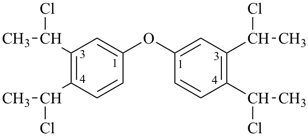
1,1′-oxybis[3,4-bis(1-chloroethyl)benzene] (PIN)
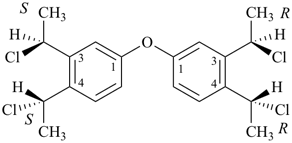
4-{3,4-bis[(1R)-1-chloroethyl]phenoxy}-1,2-bis[(1S)-1-chloroethyl]benzene (PIN)
[not 4-{3,4-bis[(1S)-1-chloroethyl]phenoxy}-1,2-bis[(1R)-1-chloroethyl]benzene;
‘R’ precedes ‘S’]
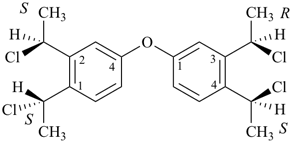
1,2-bis[(1S)-1-chloroethyl]-4-{3-[(1R)-1-chloroethyl]-4-[(1S)-1-chloroethyl]phenoxy}benzene (PIN)
(a substitutive name; a multiplicative name is not allowed)
[not 4-{3,4-bis[(1S)-1-chloroethyl]phenoxy}-2-[(1R)-1-chloroethyl]-1-[(1S)-1-chloroethyl]benzene;
‘1,2,4’ is senior to ‘4,2,1’ (see P-45.2.3)]
P-45.6.3 When names based on alphanumerical order and isotopic descriptors are the same, further choice depends on the alphabetic order of the stereochemical descriptors ‘R’ and ‘S’.
Example:
1-[(1R)-1-bromoethyl]-1-[(1S)-1-bromoethyl]cyclopentane (PIN)
[not 1-[(1S)-1-bromoethyl]-1-[(1R)-1-bromoethyl]cyclopentane;
‘R’ precedes ‘S’]
P-46 THE PRINCIPAL CHAIN IN SUBSTITUENT GROUPS
P-46.0 Introduction
P-46.1 The principal substituent chain
P-46.2 Principal substituent chains in isotopically labeled compounds
P-46.3 Principal substituent chains in compounds with stereogenic centers
P-46.0 INTRODUCTION
Compound acyclic substituents, i.e., substituted acyclic substituents, consist of a principal chain and one or more acyclic substituents. If the substituent to the principal chain also has (an) acyclic substituent(s), it itself is a compound substituent; the resulting complete substituent is called a complex acyclic substituent. Complex substituents are named by extending the methods given below for compound substituents.
Compound substituents are named in two ways:
(1) by using alkyl substituents [see P-29.2(1)];
(2) by using alkanyl substituents [see P-29.2(2)].
Alkyl and alkanyl substituent groups have been defined in Section P-29. Simple alkyl substituent groups have their free valence(s) denoted by the suffixes ‘yl’, ‘ylidene’ or ‘ylidyne’ only at position 1. Simple alkanyl substituent groups have their free valence(s) denoted by the suffixes ‘yl’ or ‘ylidene’ which may be located at any position of the chain except position 1. Both alkyl and alkanyl substituent groups can form compound substituent groups; for example, CH3-C(CH3)2– is 1,1-dimethylethyl, a compound alkyl substituent group, by method (1); and 2-methylpropan-2-yl, a compound alkanyl substituent group, by method (2). In some cases, a compound substituent group resulting from substitution of the principal chain by alkyl or alkanyl substituent groups results in the same structure that corresponds to a simple alkanyl substituent group; for example, CH3-CH2-CH2-CH2-CH(CH3)– is 1-methylpentyl by method (1), but hexan-2- yl by method (2).
P-46.1 THE PRINCIPAL SUBSTITUENT CHAIN
Selection of the principal chain of a compound substituent is accomplished in accordance with the following criteria, applied successively in the order given until a decision is reached. They are listed here and illustrated in P-46.1.1 through P-46.1.13. The principal substituent chain has:
(a) the greater number of heteroatoms; this criterion is used only in method (2) in P-46.0;
(b) the greater number of skeletal atoms, i.e., the longest chain;
| In acyclic substituents the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred acyclic substituent is the length of the chain; unsaturation is now a lower criterion [see (d)]. |
|
(c) the greater number of heteroatoms in the order: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
(d) the greater number of multiple bonds regardless of type, then the greater number of double bonds;
(e) one or more atoms with nonstandard bonding numbers;
(f) the lowest locants for heteroatoms; this criterion is used only in method (2) in P-46.0;
(g) the lowest locants for heteroatoms appearing first in the order: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
(h) the lowest locants for free valences of any kind (‘yl’, ‘ylidene’, ‘ylidyne’);
(i) the lowest locant(s) for multiple bonds, regardless of type, then the lower locant(s) for double bonds;
(j) the lowest locant(s) for (an) atom(s) with nonstandard bonding number(s);
(k) the greatest number of substituents of any kind; this criterion is applicable to both methods (1) and (2) in P-46.0;
(l) the lowest locants for substituents; this criterion is applicable to both methods (1) and (2) in P-46.0;
(m) the lowest locants for the substituent(s) cited earlier in alphanumerical order; this criterion is applicable to both methods (1) and (2) in P-46.0.
P-46.1.1 The principal substituent chain has the greater number of heteroatoms [criterion (a) in P-46.1]; this criterion is used only in method (2) of P-46.0.
Example:
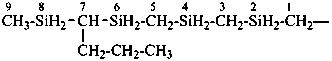
7-propyl-2,4,6,8-tetrasilanonan-1-yl (preferred prefix)
[not 7-(methylsilyl)-2,4,6-trisiladecan-1-yl]
P-46.1.2 The principal substituent chain has the greater number of skeletal atoms, i.e., the longest chain [criterion (b) in P-46.1]. This criterion is applicable to both methods (1) and (2) of P-46.0; both methods generate simple and compound substituent groups.
Examples:
 |  |
| (2) pentan-2-ylidene (preferred prefix) | (1) 1-methylbutylidene |
| (a simple substituent group) | (a compound substituent group) |
| | |
 | 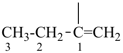 |
| (2) but-1-en-2-yl (preferred prefix) | (1) 1-methylidenepropyl |
| (a simple substituent group) | (a compound substituent group) |
| | |
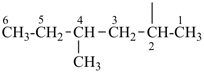 | 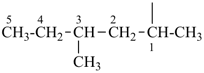 |
| (2) 4-methylhexan-2-yl (preferred prefix) | (1) 1,3-dimethylpentyl |
| (a compound substituent group) | (a compound substituent group) |
P-46.1.3 The principal substituent chain has the greater number of heteroatoms in the order: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion (c) in P-46.1].
Examples:
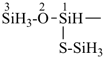
1-(silylsulfanyl)disiloxanyl (preselected prefix)
[not 1-(silyloxy)disilathianyl; ‘O’ > ‘S’]
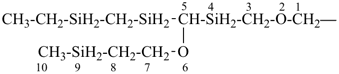
5-{[(ethylsilyl)methyl]silyl}-2,6-dioxa-4,9-disiladecan-1-yl (preferred prefix)
[not 5-[2-(methylsilyl)ethoxy]-2-oxa-4,6,8-trisiladecan-1-yl;
two oxygens are senior to one oxygen]
P-46.1.4 The principal chain has the greater number of multiple bonds regardless of type, then the greater number of double bonds [criterion (d) in P-46.1]; this criterion is applicable to methods (1) and (2) in P-46.0; both generate simple and compound substituent groups.
Examples:
 |  |
| (2) hept-1-en-4-ylidene (preferred prefix) | (1) 1-propylbut-3-en-1-ylidene |
| (a simple substituent group) | (a compound substituent group) |
| | |
 | 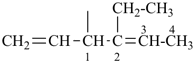 |
| (2) 4-ethylhexa-1,4-dien-3-yl | (1) 1-ethenyl-2-ethylbut-2-en-1-yl |
| (preferred prefix; a compound substituent group) | (a compound substituent group) |
P-46.1.5 The principal substituent chain has one or more atoms with nonstandard bonding numbers [criterion (e) in P-46.1]. When a choice is needed between two substituent chains having skeletal atoms with nonstandard bonding numbers, the one having the maximum number of atoms with nonstandard bonding numbers is chosen as principal substituent chain. If a further choice is needed between the same skeletal atom with different nonstandard bonding numbers, preference for the principal substituent chain is given in order of the decreasing numerical value of the bonding number, i.e., λ6 is senior to λ4.
Examples:
5-{[(methoxymethyl)sulfanyl]methyl}-4,9-dioxa-2,7λ4-dithiadecan-1-yl (preferred prefix)
[not 5-{[(methoxymethyl)-λ4-sulfanyl]methyl}-4,9-dioxa-2,7-dithiadecan-1-yl;
one nonstandard bonding atom vs. zero]
5-({[(methylsulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ4-trithiadecan-1-yl (preferred prefix)
[not 5-({[(methyl-λ4-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9- trithiadecan-1-yl;
three nonstandard bonding atoms vs. two]
5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6 ,9λ6-trithiadecan-1-yl (preferred prefix)
[not 5-({[(methyl-λ6-sulfanyl)methyl]-λ6-sulfanyl }methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
two λ6 nonstandard bonding atoms and one λ4 nonstandard bonding atoms vs. two λ4 and one λ6 nonstandard bonding atoms]
P-46.1.6 The principal substituent chain has the lowest locants for heteroatoms [criterion (f) in P-46.1]; this criterion is used only in method (2) of P-46.0.
| This is a change. Heteroatoms in chains are now considered as part of the parent hydride; as such they have seniority over suffixes for numbering (see P-14.4; see also P-15.4.3); this change makes ‘a’ terms nondetachable in skeletal replacement in chains, rings, and ring systems. |
|
Example:
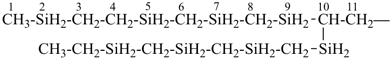
10-(1,3,5,7-tetrasilanonan-1-yl)-2,5,7,9-tetrasilaundecan-11-yl (preferred prefix)
[not 10-(1,3,5,8-tetrasilanonan-1-yl)-3,5,7,9-tetrasilaundecan-11-yl;
the
locant set in the principal substituent chain ‘2,5,7,9’ is lower than the set ‘3,5,7,9’]
P-46.1.7 The principal substituent chain has the lowest locants for heteroatoms appearing first in the order: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl [criterion(g) in P-46.1]; this criterion is used only in method (2) of P-46.0.
Example:

2-{[({[(butylsilyl)methyl]silyl}methoxy)methyl]sulfanyl}-3-oxa-5-thia-7,9-disilatridecan-1-yl (preferred prefix)
[not 2-{[({[(butylsilyl)methyl]silyl}methyl)sulfanyl]methoxy}-5-oxa-3-thia-7,9-disilatridecan-1-yl;
‘3-oxa’ is senior to ‘5-oxa’]
P-46.1.8 The principal substituent chain has the lowest locants for free valences of any kind in the order ‘yl’ > ‘ylidene’ > ‘ylidyne’ [criterion (h) in P-46.1].
Examples:
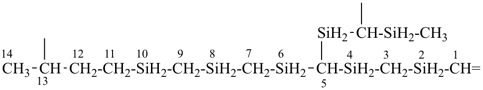
5-{[(methylsilyl)ylomethyl]silyl}-2,4,6,8,10-pentasilatetradecan-13-yl-1-ylidene (preferred prefix)
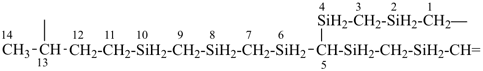
5-{[(diylomethylsilyl)methyl]silyl}-2,4,6,8,10-pentasilatetradecane-1,13-diyl (preferred prefix)
(for ‘ylo’ as a prefix, see P-70.3.1, P-71.5)
P-46.1.9 The principal substituent chain has the lowest locant(s) for multiple bonds, regardless of type, then the lowest locant(s) for double bonds [criterion (i) in P-46.1]. This criterion is applicable to both methods (1) and (2) in P-46.0; both methods generate simple and compound substituent groups.
Examples:
 | | 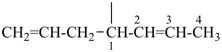 |
| (2) hepta-1,5-dien-4-yl (preferred prefix) | | (1) 1-(prop-2-en-1-yl)but-2-en-1-yl |
| (a simple substituent group) | | (a compound substituent group) |
| | | |
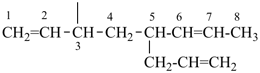 | | 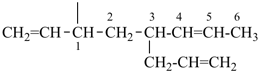 |
| (2) 5-(prop-2-en-1-yl)octa-1,6-dien-3-yl (preferred prefix) | | (1) 1-ethenyl-3-(prop-2-en-1-yl)hex-4-en-1-yl |
| (a compound substituent group) | | (a compound substituent group) |
P-46.1.10 The principal substituent chain has the lowest locant(s) for (an) atom(s) with nonstandard bonding number(s) [criterion (j) in P-46.1]. If a further choice is needed, the principal substituent chain has (an) atom(s) of the higher bonding number with the lowest locant.
Examples:
5-({[2-(methyl-λ6-sulfanyl)ethyl]sulfanyl}methyl)-4-oxa-2λ4,7λ4,10-trithiaundecan-1-yl (preferred prefix)
[not 5-({[(methylsulfanyl)ethyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7,10λ6-trithiaundecan-1-yl;
the locant set for the nonstandard bonding atoms ‘2,7’ is lower than ‘2,10’]
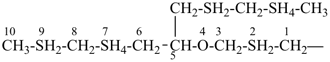
5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9λ4-trithiadecan-1-yl (preferred prefix)
[not 5-({[(methyl-λ4-sulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
the λ6 nonstandard bonding atom in the PIN name is at position ‘7’ which is lower than ‘9’]
P-46.1.11 The principal substituent chain has the greatest number of substituents of any kind [criterion (k) in P-46.1]. This criterion is applicable to both methods (1) and (2) in P-46.0; both methods generate compound and complex substituent groups, respectively.
When applicable, the principal substituent chain has the greatest number of substituent groups with the highest bonding number. When method (2) is applied, numbering is based on low locants for substituents with atoms with the highest bonding number [P-14.4 (h)].
Examples:
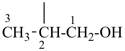 | | 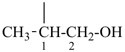 |
| (2) 1-hydroxypropan-2-yl (preferred prefix) | | (1) 2-hydroxy-1-methylethyl |
| (a compound substituent group) | | (a compound substituent group)
[not 1-(hydroxymethyl)ethyl] |
| | |
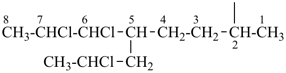 | | 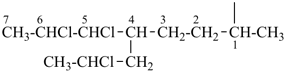 |
| (2) 6,7-dichloro-5-(2-chloropropyl)octan-2-yl (preferred prefix) | | (1) 5,6-dichloro-4-(2-chloropropyl)-1-methylheptyl |
[not 7-chloro-5-(1,2-dichloropropyl)octan- 2-yl;
a complex substituent group] | | (a complex substituent group) |
| | |
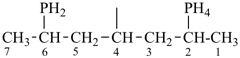 | | 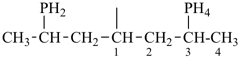 |
| (2) 2-(λ5-phosphanyl)-6-phosphanylheptan-4-yl (preferred prefix) | | (1) 3-(λ5-phosphanyl)-1-(2-phosphanylpropyl)butyl |
| (a compound substituent group) | | (a complex substituent group) |
| | |
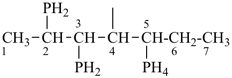 | | 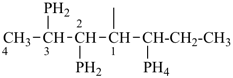 |
| (2) 5-(λ5-phosphanyl)-2,3-bis(phosphanyl)heptan-4-yl (preferred prefix) | | (1) 2,3-bis(phosphanyl)-1-[1-(λ5-phosphanyl)propyl]butyl |
| (a compound substituent group) | | (a complex substituent group) |
P-46.1.12 The principal chain has the lowest locants for substituents [criterion (l) in P-46.1]. This criterion is applicable to both methods (1) and (2) of P-46.0; both methods generate compound and complex substituent groups.
When applicable, the principal substituent chain has the greatest number of substituent groups with (an) atom(s) of the highest bonding number. When method (2) is applied, numbering is based on low locants for substituents with atoms with the highest bonding number [P-14.4 (h)].
Examples:
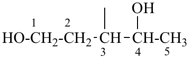 | 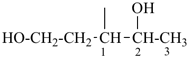 |
| (2) 1,4-dihydroxypentan-3-yl (preferred prefix) | (1) 2-hydroxy-1-(2-hydroxyethyl)propyl |
| (a compound substituent group) | (a complex substituent group)
[not 3-hydroxy-1-(1- hydroxyethyl)propyl;
the locant set ‘1,2’ is lower than ‘1,3‘] |
| | |
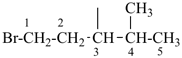 | 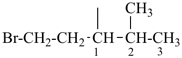 |
| (2) 1-bromo-4-methylpentan-3-yl (preferred prefix) | (1) 1-(2-bromoethyl)-2-methylpropyl |
| (a compound substituent group) | (a complex substituent group) |
| | |
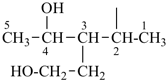 | 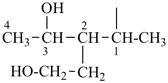 |
| (2) 4-hydroxy-3-(2-hydroxyethyl)pentan-2-yl (preferred prefix) | (1) 3-hydroxy-2-(2-hydroxyethyl)-1-methylbutyl |
| (a complex substituent group) | (a complex substituent group) |
| | |
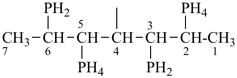 | 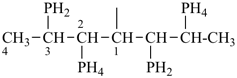 |
| (2) 2,5-bis(λ5-phosphanyl)-3,6-bis(phosphanyl)heptan-4-yl (preferred prefix) | (1) 2-(λ5phosphanyl)-3-phosphanyl-1-[2-(λ5phosphanyl)-1-phosphanylpropyl]butyl |
[not 3,6-bis(λ5-phosphanyl)-2,5-bis(phospanyl)heptan-4-yl;
the locant set ‘2,5’ is lower than ‘3,6’] | (a complex substituent group) |
P-46.1.13 The principal substituent chain has the lowest locants for the substituent(s) cited earlier in alphanumerical order [criterion (m) in P-46.1]; this criterion is applicable to both methods (1) and (2) in P-46.0; both methods generate compound and complex substituent groups.
Examples:
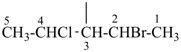 | | 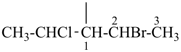 |
| (2) 2-bromo-4-chloropentan-3-yl (preferred prefix) | | (1) 2-bromo-1-(1-chloroethyl)propyl |
(a compound substituent group)
(not 4-bromo-2-chloropentan-3-yl;
‘2-bromo’ is senior to ‘4-bromo’) | | (a complex substituent group)
[not 1-(1-bromoethyl)-2-chloropropyl;
‘bromo...chloro’ is senior to ‘bromoethyl’] |
| | | |
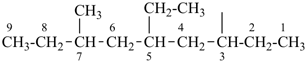 | | 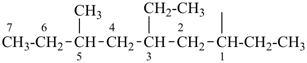 |
| (2) 5-ethyl-7-methylnonan-3-yl (preferred prefix) | | (1) 1,3-diethyl-5-methylheptyl |
| (a compound substituent group) | | (a compound substituent group) |
P-46.2 PRINCIPAL SUBSTITUENT CHAINS IN ISOTOPICALLY LABELED COMPOUNDS
P-46.2.1 The principal substituent chain contains the greater number of isotopically modified atoms.
Examples:
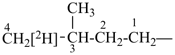
(1) 3-methyl[4-2H1]butyl (preferred prefix)
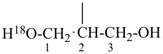
(2) 1-(18O)hydroxy-3-hydroxypropan-2-yl (preferred prefix)
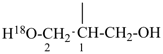
(1) 2-(18O)hydroxy-1-(hydroxymethyl)ethyl
P-46.2.2 The principal substituent chain contains the greater number of nuclides of higher mass number or isotopically modified substituent atoms or groups.
Example:
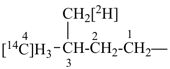
(1) 3-[2H1]methyl[4-14C]butyl (preferred prefix)
P-46.3 PRINCIPAL SUBSTITUENT CHAINS IN COMPOUNDS WITH STEREOGENIC CENTERS
P-46.3.1 The principal substituent chain contains the greater number of (Z)-double bonds.
Examples:
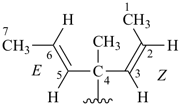 | | 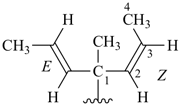 |
| (2) | | (1) |
(2) (2Z,5E)-4-methylhepta-2,5-dien-4-yl (preferred prefix)
(1) (2Z)-1-[(1E)-prop-1-en-1-yl]-1-methylbut-2-en-1-yl |
Note: The configuration at C-4 (method 2) or C-1 (method 1) cannot be given because the attached substituent is unknown. In the following example, the substitution to the parent hydride ‘siline’ creates a chiral center at C-4 (method 2) or C-1 (method 1) and a ‘R’ configuration at these positions.
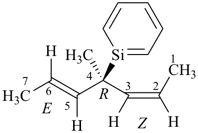 | | 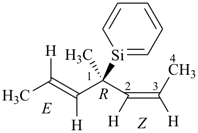 |
| (2) | | (1) |
(2) [(2Z,4R,5E)-4-methylhepta-2,5-dien-4-yl]siline (PIN)
(1) {(1R,2Z)-1-[(1E)-prop-1-en-1-yl]-1-methylbut-2-en-1-yl}siline |
P-46.3.2 The principal substituent chain contains the greater number of (R)-chirality centers.
Examples:
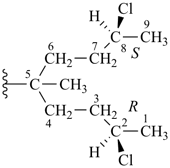 | | 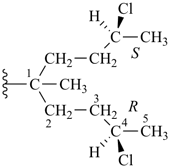 |
| (2) | | (1) |
(2) (2R,8S)-2,8-dichloro-5-methylnonan-5-yl (preferred prefix)
(1) (4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl |
Note: The configuration at C-5 (method 2) or C-1 (method 1) cannot be given because the attached substituent is unknown. In the following example, the substitution into the structure ‘trimethylsilane’ creates a chirality center at C-5 (method 2) or C-1 (method 1) and an ‘s’ configuration at these positions.
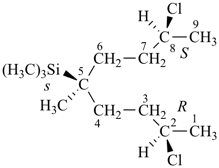 | | 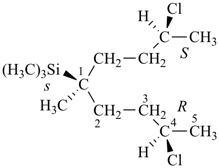 |
| (2) | | (1) |
(2) [(2R,5s,8S)-2,8-dichloro-5-methylnonan-5-yl]trimethylsilane (PIN)
(1) {(1s,4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl}trimethylsilane |
Continued with P-5 Selecting Preferred IUPAC Names and Constructing Names of Organic Compounds.
Return to:
IUPAC Chemical Nomenclature home page.
Blue Book home page.