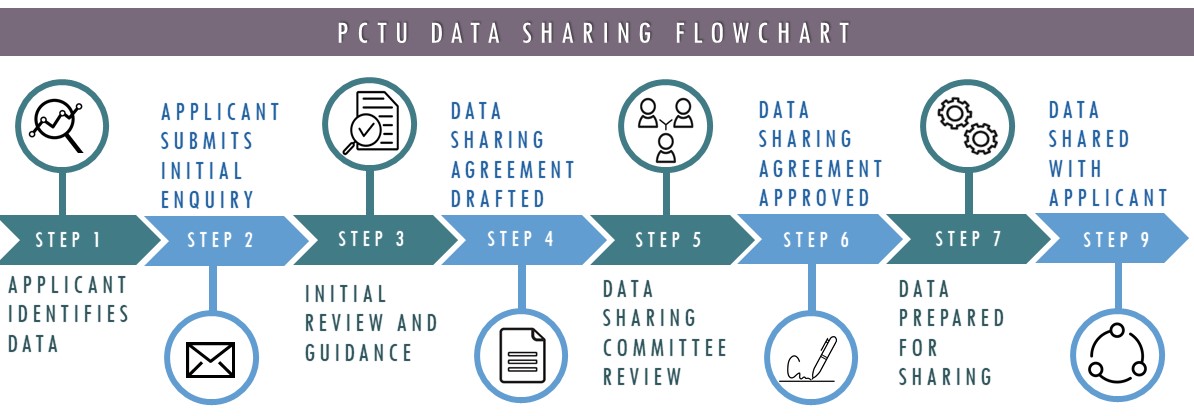
Make a Data Sharing request

Our catalogue of closed and ongoing studies provides information about all of the research carried out within the unit. If you are an external researcher that did not work on the original study and are interested in accessing data we hold, you may be interested in submitting a data sharing request.
Download our detailed information for applicants to find out more about the process and the information you will need to provide: Information for Applicants [PDF 546KB]
We recommend that you discuss your proposal with a study lead in the first instance as they will be best placed to advise on the data held and the feasibility of your request.
Information about Chief Investigators is provided on our studies page and staff working on PCTU trials are listed on our staff page.
To initiate a data sharing request, send an email to the Data Sharing Committee including information about who you have discussed your request with (i.e. the Chief Investigator, Lead Statistician or study other contact); the PCTU study (or studies) involved in your request; the purpose for requesting the data and some information about your research proposal; a broad description of the data you need; some information about your institution and the research team and any planned outputs.
Applications are assessed on case-by-case basis by the Data Sharing Committee to ensure that the proposal has scientific merit; that the data are suitable to fulfil the stated aims; that risks associated with sharing the data are minitgated and that there are sufficient assurances around data security provided by the requesting institution.
Contact the Data Sharing Committee
If you have any questions relating to data sharing or would like to discuss your proposal with a member of the team, contact the Data Sharing Committee by email and somebody will get back to your shortly.