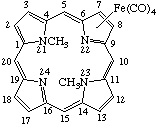
Tetracarbonyl[7-8-η-(21,23-dimethylporphyrin)]iron(0)
(η is read as hapto)
Continued from TP-8 Metal Coordination Complexes
TP-9 Metal coordination complexes - less common structural types
TP-9.1 Coordination complexes of unknown structure
TP-9.2 Coordination other than at central nitrogen
TP-9.3 Mononuclear coordination complexes with two tetrapyrrolic ligands
TP-9.4 Porphyrin as a bridging ligand
TP-9.5 Axial bridging ligands
References
Although most of the metal coordination complexes in this series fall under Rule TP-8.1, other structural types do occur, and seem likely to increase. These are named using the Rules for Coordination Compounds as illustrated below, where the number in brackets after the side heading refers to the appropriate part of those rules.
Note 1. IUPAC, Nomenclature of Inorganic Chemistry, Definitive Rules 1970 2nd ed. Butterworths, London 1971 also published in Pure Appl. Chem. 28, 39(1971), Section 7. [see also 1990 edition]Note 2. IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition Pergamon, 1979, Section D-2.
TP-9.1. Coordination Complexes of Unknown Structure (Inorganic Rule 7.41). Where the detail of structure is unknown the name, in the extended form "metal complex of free base" (e.g. the zinc coordination complex of bilirubin), indicates stoichiometric composition only.
TP-9.2. Coordination other than at Central Nitrogen (Inorganic Rules 7.33, 7.34, 7.513 and D-2.38, D-2.39.). All earlier examples assume coordination to central nitrogen atoms (Rule TP-8.1). Coordination to other sites must be specifically designated as shown below:
Example:
 |
Tetracarbonyl[7-8-η-(21,23-dimethylporphyrin)]iron(0) |
Example:
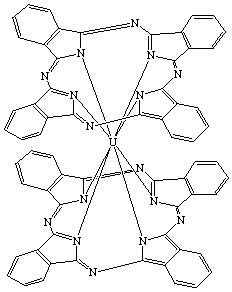 | Bis(phthalocyaninato)uranium(IV) or Uranium(IV) bisphthalocyaninate |
The bridging ligand is given the prefix μ, and if, in addition, certain atoms function as bridges, this symbol is repeated to indicate these, as shown in the following example:
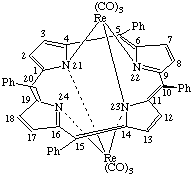 | μ-[5,10,15,20-Tetraphenylporphyrinato(2-)-N22,N24,μ-N21,μ-N23]- -bis[tricarbonylrhenium(I)] or Bis[tricarbony1rhenium(I)] μ-[5,10,15,20- |
TP-9.5. Axial Bridging Ligands.
Examples:
| 1. | 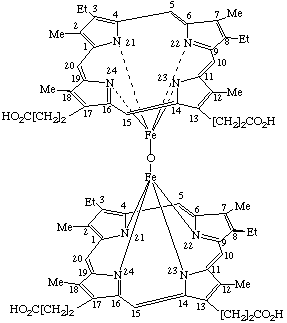 |
α,β-(μ-Oxo)-bis[mesoporphyrinatoiron(III)] |
| 2. | 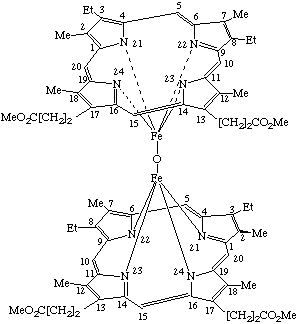 |
α,α-(μ-Oxo)-bis[(mesoporphyrinato dimethyl ester)iron(III)] or Bis[dimethyl 7,12-diethyl-3,8,13,17-tetramethyl-2,18- (Where the stereochemical configuration is unknown the stereochemical designators α and β are omitted). |
Note Because the assignment of α and β is dependent on the direction of numbering of substituents, the α side of the trivially named mesoporphyrin is the β side when named systematically.
1. R. Bonnett, Ann. N. Y. Acad. Sci., 206, 745 (1973).
2. R. Bonnett, Proceeding of 9th Meeting of the European Association for the Study of Liver Diseases, Hemsedal (1974) p. 212.
3. H. Fischer-and H. Orth, Die Chemie des Pyrrols, Volume II.1, Akademische Verlagsgessellschaft, Leipzig (1937).
4. H. Fischer and A. Stern, Die Chemie des Pyrrols, Volume II.2, Akademische Verlagsgessellschaft, Leipzig, (1940).
5. S. Sano, T. Shingu, J. M. French and E. Thonger, Biochem. J., 97, 250 (1965).
6. G. Y. Kennedy, A. H. Jackson, G. W. Kenner and C. J. Suckling, FEBS Letters, 6, 9 (1970).
7. A. W. Johnson and I. T. Kay, J. Chem. Soc., 1620 (1965).
8. R. B. Woodward, Chemical Society Meeting on Aromaticity, Sheffield, 1966. M. J. Broadhurst, R. Grigg, and A. W. Johnson, J. Chem. Soc. Perkin Trans. 1, 2111 (1972).
9. A. Eschenmoser, Chem. Soc. Quarterly Rev., 24, 366, (1970).
10. For a discussion of the confused earlier practices see T. K. With, Bile Pigments, pp. 3031, Academic Press, New York and London, 1968.
11. M. Stoll and C. H. Gray, Biochem. J., 117, 271 (1970).
12. P. O'Carra and S. D. Killilea, Tetrahedron Letters, 4211 (1970).
13. A. R. Battersby and E. McDonald, B12 (ed. D. Dolphin) Volume 1, p.107, Wiley, New York, 1982.