Fig. 24. A protohemochrome as an example of a tetrapyrrole coordination complex containing a tetrapyrrole tetradentate dianionic ligand and two pyridine molecules as axial ligands.
Continued from TP-6 and TP-7 Linear Tetrapyrroles
TP-8 Metal coordination complexes
TP-8.1 General description
TP-8.2 Coordination nomenclature
TP-8.3 Alternative order of the components of the name
TP-8.4 Trivial names
TP-8.1. General Description. The common structural pattern for metal coordination complexes in this series consists of a metal ion coordinated to a roughly planar tetrapyrrole, acting as a tetradentate dianionic ligand, and, possibly, to one or two axial ligands, as illustrated in Fig. 24.

Fig. 24. A protohemochrome as an example of a tetrapyrrole coordination complex containing a tetrapyrrole tetradentate dianionic ligand and two pyridine molecules as axial ligands.
Note See TP-8.4.3 for definition of protohemochrome .
The tetrapyrrolic ligand is oriented with the numbering clockwise when seen from above (see TP-1.2, TP-1.7 and TP-2.1). The axial ligands above and below the plane are then designated by the use of β and α respectively, in analogy with established custom for other groups of compounds. For example, hemoglobin coordinates with oxygen on the α face of the protoheme system.
Note 1. Because the assignment of α and β is dependent on the direction of numbering of substituents, they are defined only in a relative sense. For example, the α side of mesoporphyrin named systematically as 7,12-diethyl-3,8,13,17-tetramethylporphyrin-2,18-dipropionic acid is the β side of mesoporphyrin named and numbered according to the trivial system.Note 2. IUPAC-IUB Commission on Biochemical Nomenclature, "Nomenclature of Corrinoids, Rules Approved 1975". Pure Appl. Chem. 48, 495-502 (1976).
Note 3. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature. '1971 Definitive Rules for Nomenclature of Steroids." Pure Appl. Chem. 31, 284-322 (1972). [See also 1989 revision]
Coordination of the four central nitrogen atoms (N-21, N-22, N-23, N-24) is the common structural pattern, and need not be specifically designated, but may be designated as instructed in Inorganic Rule 7.33 or D-2.38 if desired. When the charge on the tetrapyrrolic ligand is not specified it is understood that it carries a formal negative charge of two units. For systems that do not conform to the common structural pattern see TP-9.2.
TP-8.2. Coordination Nomenclature. The Rules for Coordination Compounds are followed, except that stereochemical configuration of axial ligands, if any, may be designated, where known, by the use of α and β as in TP-8.1, Fig. 24. The names of ligands precede the name of the metal, the ligands being cited in alphabetical order. Enclosing marks are employed as described in Inorganic Rule 7.3 and D-2.21 of the Rules for Coordination Compounds.
Note 1. IUPAC, Nomenclature of Inorganic Chemistry, Definitive Rules 1970, 2nd ed. Butterworths, 1971 also published in Pure Appl. Chem. 28, 39 (1971), Section 7 [see also 1990 edition]; IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition, Pergamon, 1979, Section D-2.Note 2. An essential result is that organic anions, including macrocyclic anions. are placed in brackets. The names of inorganic anions are not bracketed except in special cases [e.g. when the name contains a numerical prefix e.g. (triphosphato)]. When a neutral molecule is coordinated its name is used unchanged, and is bracketed, except, on both counts, for the following common neutral ligands: water (becomes aqua), ammonia (becomes ammine), nitric oxide (becomes nitrosyl), and carbon monoxide (becomes carbonyl).
For coordination complexes of the common structural pattern decribed in Rule TP-8.1 the macrocycle name takes the ending "ato". Thus chlorin becomes chlorinato, porphyrin becomes porphyrinato, bilirubin becomes bilirubinato and mesoprophyrin diethyl ester becomes mesoporphyrinato diethyl ester. The name of the metal follows, and is itself followed by its oxidation number (Roman numeral in brackets), or its Ewens-Bassett number to indicate the charge on the entire coordination complex (instead of an oxidation number) as recommended in inorganic Rule 7.22 or Rule D-2.23.
Note 1. The designation of the charge on the ligand such as (2-) is often not specified.Note 2. See Inorganic Rule 2.252 for definition.
Examples: (Py = pyridine)
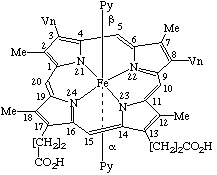
| 1. | 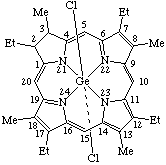 | Dichloro(2,7,12,17-tetraethyl-2,3-dihydro-3,8,13,18- -tetramethylporphyrinato)germanium(IV) |
| 2. | 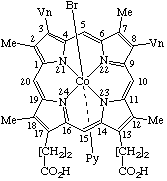 | β-Bromo(protoporphyrinato dimethyl ester)-α-(pyridine)cobalt(III) or β-Bromo[dimethyl 3,7,12,17-tetramethyl-8,13-divinylporphyrin- -2,18-dipropionato(2-)]-α-(pyridine)cobalt(III) |
| 3. | 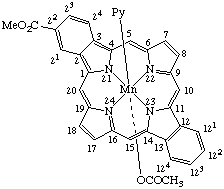 | α-(Acetato)[22-(methoxycarbonyl)dibenzo[b,l]-5-azaporphyrinato]- -β-(pyridine)manganese(III) |
| 4. | 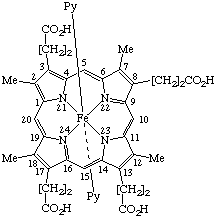 | (Coproporphyrinato III)bis(pyridine)iron(II) or Bis(pyridine)(3,8,13,17-tetramethylporphyrin-2,7,12,18- -tetrapropionato)iron(II) |
Note Practice varies as to whether the protons of the acid groups are formally removed from the ligand or are retained in naming such coordination complexes. If they are retained for this coordination complex, the name is based on iron. If they are removed, a hexaanionic ligand results, and the precise name of the anion would be [coproporphyrinato(6-) III N21,N22,N23,N24]bis(pyridine)ferrate(4–).
| 5. | 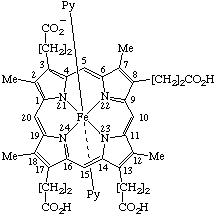 | [Coproporphyrinato(6–) III)bis(pyridine)iron(III) or Bis(pyridine)[3,8,13,17-tetramethylporphyrin-2,7,12,18- -tetrapropionato-(3-)]iron(III) |
The axial ligands are cited in alphabetical order and with enclosing marks as before (Rule TP-8.2.). The name of the metal follows, and is itself followed by the oxidation number (Roman numeral in parentheses) or the Ewens-Bassett number. After a space the name of the tetrapyrrole ligand is given, ending in "ate": it is not bracketed.
Note The use of the "ate" ending here is not sanctioned by any current organic nomenclature rule in IUPAC Sections A, B, C, D or in the IUPAC inorganic rules. In the organic rules, "ate" refers to an anion derived by loss of a proton from a functional group (see IUPAC Rules C-84.1 and C-84.2). In the inorganic nomenclature rules "ate" denotes the loss of a proton from an acid group, or the presence of an anionic coordination center (see Inorganic Rule 3.223).
The structures drawn in Rule TP-8.2. are named as follows on the basis of this alternative procedure:
1. Dichlorogermanium(IV) 2,7,12,17-tetraethyl-2,3-dihydro-3,8,13,18-tetramethylporphyrinate2. β-Bromo-α-(pyridine)cobalt(III) protoporphyrinate dimethyl ester
3. α-(Acetato)-β-(pyridine)manganese(III) 22-(methoxycarbonyl)dibenzo[b,l]-5-azaporphyrinate
4. Bis(pyridine)iron(II) coproporphyrinate III
5. Bis(pyridine)iron(III) coproporphyrinate(3-) III
TP-8.4. Trivial Nanes. Because of their natural occurrence the magnesium and iron coordination complexes are associated with an extensive trivial nomenclature.
TP-8.4.1. Nine trivial names may be used to name the chlorophyll structures as indicated in Fig. 25, Fig. 26, Fig. 27, and Fig. 28.
Fig. 26. Chlorophyll c1 R = Et
Fig. 27. Bacteriochlorophyll a R = phytyl.
Fig. 28. Bacteriochlorophyll b R = Phytyl.
Note 2. The structure of bacteriochlorophyll b has been formulated by H. Scheer, W. A. Svec, B. T. Cope, M. H. Studier, R. G. Scott and J. J. Katz, J. Am. Chem. Soc. 96, 3714 (1974).
TP-8.4.2. Alternatively, the structures represented in Fig. 25, Fig. 27 and Fig. 28 may be named semisystematically as magnesium(II) coordination complexes of pheophytins, bacteriopheophytins or mesopheophytin, respectively. Chlorophyll c1 and c2 may be named semisystematically as 31,32,171,172-tetradehydro-132-(methoxcarbonylphytoporphyrinato) and 31,32,81,82,171,172-hexadehydro-132-(methoxycarbonyl)phytoporphyrinato magnesium(II) coordination complexes, respectively. Chlorophyll structures containing a free propionic acid residue at position C-17 are called chlorophyllides. They may be named semisystematically as magnesium(II) coordination complexes of pheophorbides.
TP-8.4.3. Similarly terms for iron coordination complexes may be defined and interrelated as follows:
Iron Coordination Complexes
Note Such compounds appear to be isolated as the μ-oxo anhydro dimers (μ-oxo dimers). Examples are given in Rule TP-9.5.
Examples:
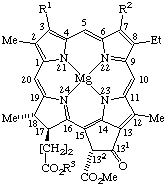
Fig. 25. Chlorophyll a R1 = Vn R2 = Me R3 = phytyl Chlorophyll b R1 = Vn R2 = -CHO R3 = phytyl Chlorophyll d R1 = -CHO, R2 = Me R3 = phytyl Mesochlorophyll a R1 = Et R2 = Me R3 = phytyl Chlorophyllide a R1 = Vn R2 = Me R3 = -H. Note Phytyl = (2E)-(7R,11R)-3,7,11,15-tetramethyl-2-hexadecenyl

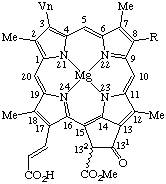
Chlorophyll c2 R = Vn.Note The double bond at C-171 is E which is implied by these names. However, if the absolute configuration is known at C-132, it may be expressed by R or S placed in front of these names. See TP-0.3.
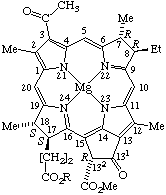
Note Bacteriochlorophyllide a (all-trans)-geranylgeranyl ester has also been reported, and called bacteriochlorophyll gg: J. J. Katz, H. H. Strain, A. L. Harkness, M. H. Studier, W. A. Svec, T. R. Janson and B. T. Cope, J. Am. Chem. Soc. 94, 7938 (1972).
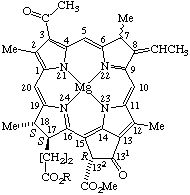
Note 1. The stereochemistry of the 8(81) double bond may be designated by E or Z in accord with TP-0.3.
Note 1. See TP-4.3.2 for pheophytin and pheophorbide names.
heme : an iron porphyrin coordination complex ferroheme : an iron(II) porphyrin coordination complex ferriheme : an iron(III) porphyrin coordination complex hemochrome : a low-spin iron porphyrin coordination complex with one or more strong field axial ligands
(e.g. pyridine)ferrohemochrome : an iron(II) hemochrome ferrihemochrome : an iron(III) hemochrome hemin : a chloro(porphyrinato)iron(III) coordination complex. For example protohemin =
chloro(protoporphyrinato)iron(III) or chloroiron(III) protoporphyrinatehematin : a hydroxo(porphyrinato)iron(III) coordination complex.
| 1. | 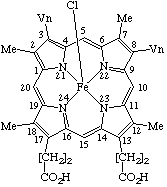 | Protohemin or Chloro(protoporphyrinato)iron(III) |
| 2. |  | Bis(pyridine)coproferrohemochrome III or (Coproporphyrinato III)bis(pyridine)iron(II) |