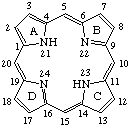
Fig. 2. Porphyrin 1-24 Numbering Scheme.
Continued from Introduction
TP-0 General considerations
TP-1 Fundamental Porphyrin Systems
TP-1.1 Porphyrin ring system
TP-1.2 Numbering
TP-1.3 Additional fused rings
TP-1.4 Skeletal replacement
TP-1.5 Skeletal replacement of nitrogen atom
TP-1.6 Fused porphyrin replacement analogs
TP-1.7 Systematic names for substituted porphyrins
TP-0.1. The established international rules for naming organic compounds and natural products apply to tetrapyrroles, except where otherwise stated. See IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979.
TP-0.3. Unless implied by a trivial name, the absolute configuration of a tetrahedral chiral center is designated by an R or S symbol assigned by the sequence-rule procedure. Similarly, the stereochemistry about a double bond is denoted by the prefixes E or Z. The symbols are assigned in accord with procedures given in the international recommendations for stereochemistry and are normally cited with their corresponding locants, when necessary, in front of the whole name. Inversion of configuration or change in the configuration of a double bond from that implied by a name is expressed in front of the name in accord with IUPAC Rule F-6.3. [see RF-10.2 in the 1999 revision.]
Note IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979, Rules E-2.2 and E-4.9.
Rule Tetrapyrroles 1. Fundamental Porphyrin Systems
TP-1.1. Porphyrin ring system. The fundamental macrocyclic tetrapyrrolic ring system shown in Fig. 2 is named porphyrin. It is tautomeric with respect to the location of the two hydrogen atoms not involved in the peripheral conjugated system. The two hydrogen atoms may be associated with any two of the four nitrogen atoms. Unless stated to the contrary the representation of one tautomeric form does not imply the absence of another tautomer. However, for nomenclature purposes, the name "porhyrin" implies that the saturated nitrogen atoms are at positions 21 and 23 unless specifically indicated otherwise.
Note The name "porphine" has also been used widely for the structure in Fig. 2. For further discussion see A. H. Corwin in Organic Chemistry. (H. Gilman, ed.) Vol. 2. Wiley N. Y., 1943, p. 1272 and reference [ref 1].

Fig. 2. Porphyrin 1-24 Numbering Scheme.
TP-1.2. Numbering. The 1-24 numbering system upon which the numbering system for corrinoids is based is adopted for the porphyrin nucleus and is shown in Fig. 2. The 2,3,7,8,12,13,17 and 18 positions have commonly been referred to generically as "beta-positions" (i.e. of the pyrrole rings). Similarly, positions at 1,4,6,9,11,14,16 and 19 have been referred to generically as "alpha-positions," while those at 5,10,15 and 20 are referred to generically as "meso-positions." However, in order to avoid possible ambiguity with stereochemical designations the use of these generic terms is discouraged.
See IUPAC-IUB Commission on Biochemical Nomenclature, "Nomenclature of Corrinoids, Rules Approved 1975." Pure Appl. Chem. 48, 495-502 (1976).
TP-1.3. Fused Systems. Compounds with extra rings ortho-fused or ortho-perifused to the porphyrin nucleus are of some importance. Tetrabenzoporphyrin is defined and the extra positions numbered as shown in Fig. 3. The name may be used without fusion letters to refer to tetrabenzo[b,g,l,q]porphyrin. Systems in which the porphyrin nucleus is fused with other cyclic hydrocarbons may be named by analogy to the IUPAC Rules B-3 and A-21 for naming fused ring systems, but adopting the porphyrin nucleus with the implied hydrogen atoms at positions 21 and 23 as the basic component to which other rings are fused. Fusion locants are derived according to IUPAC Rule A-21.5. Indicated hydrogen is determined on the basis of the presence of implied hydrogen atoms at positions 21 and 23 of the porphyrin ring in contrast to the form containing the maximum number of noncumulative double bonds as in IUPAC Rule A-21.6.
Note 1 See also TP-3.4 for use of "cyclo".Note 2 See TP-1.6 for fused systems involving skeletally replaced porphyrins.
Note 3 Fusion of the porphyrin ring system with heterocyclic rings, particularly one containing a bivalent atom may affect the tautomerism of the nitrogen atoms of the porphyrin ring system and needs further consideration.
The component rings fused to the porphyrin base are numbered as substituent groups of the lowest possible position of the porphyrin ring. However, where the "extra" rings are formally derived, such as for lactones, from the reaction of noncyclic substituents implied by the trivial name the numbering of the noncyclized substituent is retained as illustrated by example 4 of Rule TP-3.1 and the examples of Rule TP-3.4.
Note See TP-2.1 Fig. 6. This practice is in contrast to established procedures of systematic organic nomenclature for orienting and numbering fused rings according to IUPAC Rules A-22 and B-3.4.
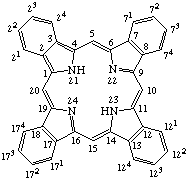
Fig. 3. Tetrabenzoporphyrin.
Examples:
| 1. | 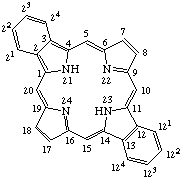 | Dibenzo[b,l]porphyrin |
| 2. | 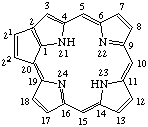 | Cyclopenta[at]porphyrin |
Note The name phorbine has been used for the tetrahydro derivative of this ring system, numbered and oriented as follows:
Phorbine
| 3. | 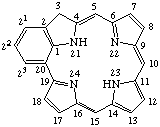 | 3H-Benzo[at]porphyrin |
Note 1 See TP-3.5 for skeletal replacement of substitued porphyrins.Note 2 These rules have been developed in order to name the common replacement analogs. However further refinement is needed in order to name replacement analogs of other porphyrin types and for corrins and corroles.
Examples:
| 1. | 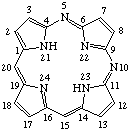 | 5,10-Diazaporphyrin |
| 2. | 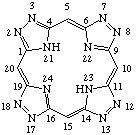 | 2,3,7,8,12,13,17,18-Octaazaporphyrin |
| 3. | 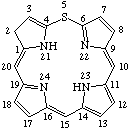 | 2H-5-Thiaporphyrin |
| 4. | 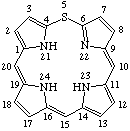 | 24H-5-Thiaporphyrin |
| 5. | 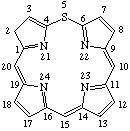 | 21,23-Didehydro-2H-5-thiaporphyrin |
| 6. | 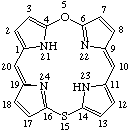 | 5-Oxa-15-thiaporphyrin |
Note Systematic organic nomenclature requires that the parent upon which replacement is performed be a hydrocarbon (see IUPAC Rules B-4, C-0.6 and D-1.6). Thus, the replacement operation is generally limited to replacement of carbon atoms by atoms of other elements. For natural products IUPAC provisional rules F-4.11 to F-4.13 permit replacement of carbon atoms of a heterocyclic parent, and also the replacement of noncarbon atoms by carbon atoms. The replacement of noncarbon atoms by other noncarbon elements is introduced for porphyrin nomenclature, but needs further consideration.
| 1. | 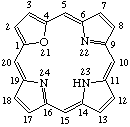 | 23H-21-Oxaporphyrin |
| 2. | 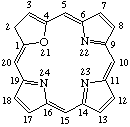 | 2H-21-Oxaporphyrin |
| 3. | 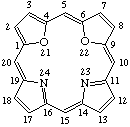 | 21,22-Dioxaporphyrin |
| 4. | 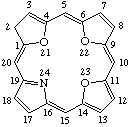 | 2H-21,22,23-Trioxaporphyrin |
| 5. | 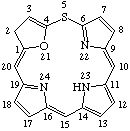 | 2H,23H-21-Oxa-5-thiaporphyrin |
Note This is in contrast to the procedures for systematic organic nomenclature in which the fusion operation is performed before skeletal replacement is carried out. See IUPAC Rule B4.2.
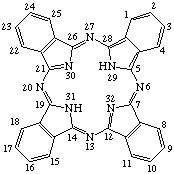
Fig. 4. Phthalocyanine.
Example:
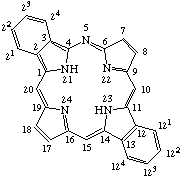 | Dibenz[b,l]-5-azaporphyrin |
Note 1. IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979.Note 2. But see also TP-4 for nomenclature of reduced porphyrins including chlorins.
Substituted porphyrins named systematically are numbered by the rules for systematic organic nomenclature, IUPAC Rule C-15.1. When there is a choice the starting point and direction of numbering around the porphyrin nucleus are chosen so as to give lowest locants to the following structural factors (if present), considered successively in the order listed until a decision is reached:
(a) hydrogen atoms expressed as "indicated hydrogen"
(b) principal groups named as suffix
(c) substituents named as prefixes and hydro prefixes all considered together in one series in ascending numerical order
(d) the substituent named as a prefix, which is cited first in a name.
Note Because the name "porphyrin" implies a "dihydro parent," which contains two more hydrogen atoms than the corresponding fully conjugated ring system, the notation of indicated hydrogen (a locant and a capital italic H), introduced in IUPAC Rule A-21.6, is adapted to denote one of several possible isomers of a particular parent porphyrin structure or a particular tautomer. See also TP-1.4.
Examples:
| 1. | 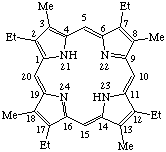 | 2,7,12,17-Tetraethyl-3,8,13,18-tetramethylporphyrin |
| 2. | 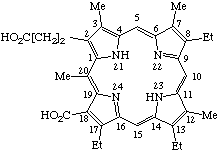 | 18-Carboxy-8,13-diethyl-3,7,12,17,20-pentamethylporphyrin- -2-propionic acid |
| 3. | 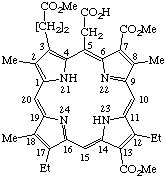 | 12,17-Diethyl-7,13-bis(methoxycarbonyl)-3-[2- -(methoxycarbonyl)ethyl]-2,8,18-trimethylporphyrin- -5-acetic acid |
Note In line with IUPAC Rule C-10.3, a carboxylic acid is preferred to an ester. Thus the parent, porphyrin-5-acetic acid, is preferred to methyl porphyrin-3-propionate.
| 4. | 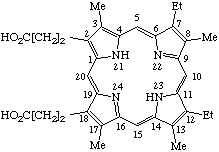 | 7,12-Diethyl-3,8,13,17-tetramethylporphyrin- -2,18-dipropionic acid |
| 5. | 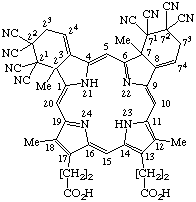 | 21,21,22,22,71,71,72,72-octacyano-2,21,22,23,7,71,72,73- -octahydro-2,7,12,18-tetramethyldibenzo[b,g]porphyrin- -13,17-dipropionic acid |