A. General Considerations
Glycolipids (a contraction of glycosyllipids) are generally named as glycosyl derivatives of the corresponding lipid, e.g., diacylgalactosyIglycerol, glucosylceramide. Because of the heterogeneity of the fatty acids and long-chain bases encountered in most cases, a generic name for the lipid moiety is needed, i.e. ceramide. With higher glycosphingolipids, especially the gangliosides, naming problems arise from the complexity of the carbohydrate moiety of these compounds. The systematic names of the oligosaccharides linked to ceramide are so cumbersome that they are of the same practical value as, e.g., the systematic name for a peptide hormone such as insulin. It was felt that this difficulty could be overcome only by creating suitable trivial names for some parent oligosaccharides. In constructing these names (see Table 1) the following principles were observed:
(a) The number of monosaccharide units in an oligosaccharide is indicated by the suffixes '-biose', '-triaose', 'tetraose', etc. This follows the well established practice in the carbohydrate field (cf. cellobiose, cellotetraose, maltotetraose, etc.), with the exception that the suffix '-triose', as used in maltotriose, has been changed to 'triaose' to avoid confusion with the monosaccharides called trioses.
(b) The oligosaccharides are grouped in series according to their structure and biogenetic relationship.
(c) Differences in linkage (e.g., 14 versus 1
3) in otherwise identical sequences are indicated by 'iso-' or 'neo-', used as a prefix. On the basis of these names, the semisystematic nomenclature for neutral glycosphingolipids and gangliosides described below is recommended. A set of symbols has been devised that allows a simple representation of complex neutral and acidic glycosphingolipids (Table 1).
Table 1. Names and abbreviations of simple glycolipids
| Structurea | Trivial name of oligosaccharideb | Symbolc | Short symbold |
|---|---|---|---|
| Gal(a1-4)Gal(b1-4)GlcCer | Globotriaose | GbOse3 | Gb3 |
| GalNAc(b1-3)Gal(a1-4)Gal(b1-4)GlcCer | Globotetraose | GbOse4 | Gb4 |
| Gal(a1-3)Gal(b1-4)GlcCer | Isoglobotriaose | iGbOse3 | iGb3 |
| GalNAc(b1-3)Gal(a1-3)Gal(b1-4)GlcCer | Isoglobotetraose | iGbOse4 | iGb4 |
| Gal(b1-4)Gal(b1-4)GlcCer | Mucotriaose | McOse3 | Mc3 |
| Gal(b1-3)Gal(b1-4)Gal(b1-4)GlcCer | Mucotetraose | McOse4 | Mc4 |
| GlcNAc(b1-3)Gal(b1-4)GlcCer | Lactotriaose | LcOse3 | Lc3 |
| Gal(b1-3)GlcNAc(b1-3)Gal(b1-4)GlcCer | Lactotetraose | LcOse4 | Lc4 |
| Gal(b1-4)GlcNAc(b1-3)Gal(b1-4)GlcCer | Neolactotetraose | nLcOse4 | nLc |
| GalNAc(b1-4)Gal(b1-4)GlcCer | Gangliotriaose | GgOse3 | Gg3 |
| Gal(b1-3)GalNAc(b1-4)Gal(b1-4)GilcCer | Gangliotetraose | GgOse4 | Gg4 |
| Gal(a1-4)GalCer | Galabiose | GaOse2 | Ga2 |
| Gal(l-4)Gal(a1-4)GalCer | Galatriaose | GaOse3 | Ga3 |
| GalNAc(1-3)Gal(1-4)Gal(a1-4)GalCer | N-Acetylgalactosaminyl- galatriaose | GalNAc1-3GaOse3 | - |
b Name of glycolipid is formed by converting ending '-ose' to '-osyl', followed by '-ceramide', without space; e.g., globotriaosylceramide.
c Should be followed by Cer for the glycolipid, without space; e.g., McOse3Cer, Mc4Cer (see Lip-3.13).
d The short form should be used only in situations of limited space or in case of frequent repetition.
B. Generic Terms
Lip-3.1. The term 'glycolipid' designates any compound containing one or more monosaccharide residues linked by a glycosyl linkage to a lipid part [e.g., a mono- or diacylglycerol, a long-chain base (sphingoid) like sphingosine, or a ceramide].
Lip-3.2. The term 'glycoglycerolipid' may be used to designate glycolipids containing one or more glycerol residues.
Lip-3.3. The term 'glycosphingolipid', as hitherto, includes all compounds containing at least one monosaccharide and a sphingoid. The glycosphingolipids can be subdivided as follows:
Neutral glycosphingolipids: monoglycosyl- and oligoglycosylsphingoids; monoglycosyl- and oligoglycosylceramides.
Acidic glycosphingolipids: sialosy1glycosylsphingolipids (gangliosides); sulfoglycosylsphingolipids (formerly 'sulfatides', which is not recommended) (cf. Lip-3.11).
Lip-3.4. 'Psychosine' may be used as a generic name for 1-monoglycosylsphingoids, although the latter is preferred. The nature of the monosaccharide and the sphingoid is not specified in this name.
Lip-3.5. The term 'fucolipid' may be used to designate fucose-containing neutral or acid glycolipids.
C. Individual Compounds
Note. 'Individual' in this section refers to the carbohydrate moiety only.
Lip-3.6. Glycoglycerolipids may be named either as glycosyl compounds according to Rule Carb-24 or as glycosides according to Rule Carb-23 [5].
Example: the compound XVIII may be named either 1,2-diacyl-3-b-D-galactosyl-sn-glycerol or 1,2-diacyl-sn-glycerol 3-b-D-galactoside.
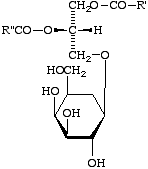
XVIII
Comment. The first form is preferred, as the glycosphingolipids are also named this way.
Lip-3.7. A glycosphingolipid is generally named as a 'glycosylsphingoid' or a 'glycosylceramide', using the appropriate trivial name of the mono- or oligosaccharide residue for 'glycosyl'. It is understood that the sugar residue is attached to the C-1 hydroxyl group of the ceramide. For glycosphingolipids carrying two to four saccharide residues, the trivial names listed in Table 1 are recommended.
Comment. It is strongly recommended that the name of the oligosaccharide be defined in each publication by means of the standard symbols for sugars (as in Table 1, column 1) rather than by the full name, which is often so long as to be confusing.
Lip-3.8. The trivial name 'cerebroside' designates 1-b-glycosylceramide (the natures of the sphingoid and of the fatty acid are not specified in this name).
Lip-3.9. Glycosphingolipids carrying fucose either as a branch or at the end of an oligohexosyleeramide are named as 'fucosyl(X)osylceramide' where (X) stands for the root name of the oligosaccharide. The location of the fucosyl residue is indicated by a Roman numeral designating the position of the monosaccharide residue in the parent oligosaccharide (counting from the ceramide end) to which the fucose residue is attached, with an Arabic numeral superscript indicating the position within that residue to which the fucose is attached. If necessary, the anomeric symbol can be used as usual, i.e. preceding 'fucosyl-'.
Examples for Lip-3.7 and Lip-3.9 (structures given in the symbols of Lip-3.13):
(a) lactosylceramide for Gal (b14)GlcCer;
(b) mucotriaosylceramide for Gal(b14)Gal(b1
4)GlcCer;
(c) III2-a-fucosylisoglobotriaosylceramide for Fuc(a12)Gal(a1
3)Gal(b1
4)Glc(b1
1)Cer.
Note. D is omitted by convention in the abbreviated formulas, but D (or L) may be inserted when desirable. Hyphens may replace left-to-right arrows (see section 3.4 of [13]).
Lip-3.10. Sialoglycosphingolipids (synonym: gangliosides) are glycosphingolipids carrying one or more sialic residues. Sialic acid is the generic term for N-acetyl- or N-glycoloylneuraminic acid (cf. section 3 in [1]). Gangliosides are named as N-acetyl- (or N-glycoloyl-)neuraminosyl-(X)osylceramide, where (X) stands for the root name of the neutral oligosaccharide to which the sialosyl residue is attached (cf. Table 1). The position of the sialosyl residue is indicated in the same way as in the case of fucolipids (see Lip-3.9).
Example: II3-N-acetylneuraminosyllactosylceramide for AcNeu(a23)Gal(b1
4)Glc(b1
1)Cer.
Lip-3.11. Glycosphingolipids carrying a sulfuric ester (sulfate) group, formerly called sulfatides, are preferably named as sulfates of the parent neutral glycosphingolipid. The location of the sulfate group may be indicated as in Lip-3.9.
Example: lactosylceramide II3-sulfate.
Lip-3.12. Phosphoglycosphingolipids with phosphodiester structures are named according to the recommendation for the phospholipids (see above).
D. Symbols and Abbreviations
Lip-3.13. Simple or complex glycosphingolipids can be represented according to existing rules, using the symbols Cer, Sph, AcNeu, etc. (Appendix B), together with the recommended [13] symbols for the hexoses (Glc, Gal, etc.). Examples are given above, and in Table 1 and Appendix C. However, due to the complexity of the higher glycosphingolipids, this often results in very long and cumbersome series that are not easy to comprehend. It is therefore recommended that the oligosaccharides listed in Table 1 be represented by specific symbols in which the number of monosaccharide units (-oses) is indicated by Osen, preceded by two letters representing the trivial name of the oligosaccharide (column 3). For a short form, which may be required in the case of limited space or frequent repetition, Ose can be omitted (column 4); however, the long form is preferred as being more evocative.
Examples:
(a) McOse3Cer for mucotriaosylceramide, Gal(b1-4)Gal(b1-4)Glc(1-1)Cer;
(b) II3 AcNeuGgOse4Cer for II3-N-acetylneuraminosylgangliotetraosylceramide,
Galb13GalNAcb1
4Gal(3
2-aNeuAc)b1
4Glcb1
1Cer (see Lip-3.14 for this mode of representing a branched chain).
Abbreviations for the more important gangliosides are given in Appendix C.
Lip-3.14. When it is desirable to represent a branched oligosaccharide on a single line, as in running text or a table, the parentheses surrounding the locants in the main chain may be omitted and used instead to enclose the symbols for the branched portion(s) of the molecule. The branches follow, in parentheses and with appropriate arrows, the residues to which they are attached.
Examples:
(a) NeuGca23Galb1
3GalNAcb1
4Gal(3
2aNeuGc)b1
4Glcb1
1Cer;
(b) NeuAca23Galb1
3GalNAcb1
4Gal(3
2aNeuAc8
2aNeuAc)b1
4Glcb1
1Cer;
(c) GalNAca1g3Gal(2
1aFuc)b1
4GlcNAc(3
1aFuc)b1
3Galb1
4Glcb1
1Cer
III3,IV2-a,a-difucosyl-IV3-a-2-acetamido-2-deoxgalactosylneolactotetraosylceramide
III3,IV2 (Fuca)2,IV3GalNAca-nLcOse4Cer.
IV. NEURAMINIC ACID
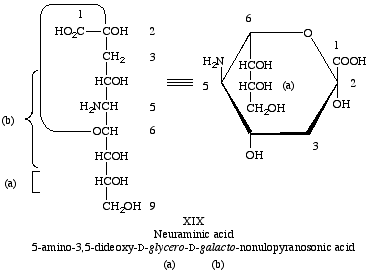
Lip-4.1. The compound 5-amino-3,5-dideoxy-D-glycero-D-galacto-nonulosonic acid is neuraminic acid (XIX), with the symbol Neu [11].
Lip-4.2. The term 'sialic acid' signifies the N-acylneuraminic acids and their esters and other derivatives of the alcoholic hydroxyl groups.
Lip-4.3. The radicals resulting from the removal of a hydroxyl group of neuraminic acid or sialic acid are designated as neuraminoyl or sialoyl, respectively, if the hydroxyl is removed from the carboxyl group, and as neuraminosyl and sialosyl, respectively, if the hydroxyl group is removed from the anomeric carbon atom of the cyclic structure.
REFERENCES
1. IUPAC-IUB Commission on Biochemical Nomenclature (1967) Eur. J. Biochem. 2, 127-131, also 12, 1 (1970); [see also Biochemistry, 6, 3287-3292 (1967); Biochem. J., 105, 897-902 (1967); J. Biol. Chem., 242, 4845-4849 (1967); Hoppe-Seyler's Z. Physiol. Chem., 350, 279-285 (1969) (in German)].
2. International Union of Pure and Applied Chemistry (1973) Information Bulletin, Appendix 31. [See also Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, 1979. Edited by J Rigaudy and S P Klesney.]
3. International Union of Biochemistry (1966) Nomenclature of Organic Chemistry (Sections A, B and C) 2nd edn, Butterworths, London. [See also Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, 1979. Edited by J Rigaudy and S P Klesney.]
4. Mills, J. A. & Klyne, W. (1954) Progr. Stereochem. 1, 181.
5. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1971) Eur. J. Biochem. 21, 455-477, also 25, 4 (1972) [now revised as Nomenclature of Carbohydrates (1996), Carb-23 and Carb-24 are now 2-Carb-33.2, 2-Carb-31.2 and 2-Carb-33.6].
6. International Union of Pure and Applied Chemistry (1970) J. Org. Chem. 35, 2849-2867; also Eur. J. Biochem. 18, 151-170 (1971) [now revised as Nomenclature of Organic Chemistry: Section E].
7. Baer, E. & Fischer, H. O. L. (1939) J. Biol. Chem. 128, 475.
8. Baddiley, L, Buchanan, J. G. & Carss, B. (1957) J. Chem. Soc. 1869.
9. Hirschmann, H. (1960) J. Biol. Chem. 235, 2762.
10. IUPAC-IUB Commission on Biochemical Nomenclature (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 2222-2230; Hoppe-Seyler's Z. Physiol. Chem. 358, 599-616 (1977); also Eur. J. Biochem. 79, 1-9 (1977) [see also Biochem. J., 1978, 171, 1-19; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 256-264 (Nomenclature of phosphorus-containing compounds of biochemical importance)].
11. IUPAC-IUB Commission on Biochemical Nomenclature (1972) Eur. J . Biochem. 27, 201-207 [also in Biochem. J., 126, 92-97 (1972); Biochim. Biophys. Acta, 263, 205-212 (1972); J. Biol. Chem., 247, 977-983 (1972); Rev. Soc. Quim. Mex., 19, 33-38 (1975); now revised and incorporated in Nomenclature and Symbolism for Amino Acids and Peptides (1983)].
12. IUPAC-IUB Commission on Biochemical Nomenclature (1970) Eur. J. Biochem. 15, 203-208 [see also Arch. Biochem. Biophys. 1971, 145, 425-436; Biochem. J., 1971, 120, 449-454; Biochemistry, 1971, 9, 4022-4027; Biochim. Biophys. Acta 1971, 247, 1-12; 1972, 25, 1; J. Biol. Chem., 1970, 245, 5171-5176; J. Mol. Biol., 1971, 55, 299-310; Pure Appl. Chem., 1974, 40, 277-290; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 109-114].
13. IUPAC-IUB Combined Commission on Biochemical Nomenclature (1967) Eur. J. Biochem. 1, 259-266 [see also Bull. Soc. Chim. Biol. 50, 3-20 (1968) (in French)].
Return to main IUPAC nomenclature home page
Return to main IUBMB nomenclature home page
Return to Biochemical Nomenclature Committees home page