Nomenclature of Lipids
No Longer Recommended
Lip-1.10
Continued from suplement
The recommendations on the Nomenclature of Glycolipids imply that parts of Lip-1.10 are no longer recommended. The following is the full original text. As a consequence some alternative names quoted elsewhere in the document have been omitted from the web version. These too are listed below.
Lip-1.10. Affixes denoting substitution of sphinganine (hydroxy, oxo, methyl, etc.) are used as usual, according to existing rules [3]. The configurations of additional substituents should be specified by the prefixes 'D' or 'L' (italic capitals, cf. [4]), following the locant of substitution. These prefixes refer to the orientation of the functional groups to the right or left, respectively, of the carbon chain when written vertically in a Fischer projection with C-1 at the top (cf. Formulae I-VI). If the configuration is unknown, the prefix 'X' may be used. In the case of a racemic mixture, 'rac' should be used as a prefix to the name.
Sphingoids differing from sphinganine in their configurations at C-2 and/or C-3 should be named not as derivatives of sphinganine, but with fully systematic names [3], using the prefixes D-threo, L-erythro, as appropriate, e.g., D-threo-2-amino-1,3-octadecanediol, or (2R,3R)-2-amino-1,3-octadecanediol, for II (cf. Rule Carb-8 in [5]). (Cf. Lip-1.11, example [d]).
Comments. (a) The semisystematic names for the sphingoids are significantly shorter than the fully systematic names only if the terms chosen imply not only substituents but also configurations. Therefore, the name 'sphinganine' specifies the D-erythro configuration, and it is logical that the names of stereoisomers of sphinganine differing in configuration at C-2 and/or C-3 should not include 'sphinganine' as a root. This recommendation differs from that in the previous document [1].
(b) The configurations usually encountered have identical configurational prefixes only if a D/L but not if the R/S system [6] is used; e.g., C-3 is D and R in icosasphinganine (III) and D and S in 4D-hydroxysphinganine (IV). Whenever it is desirable to use the R/S system, the fully systematic name should be used with the specification of configuration at every center (and, when applicable, of the configuration at the double bond).
Examples:
(a) (2R,3R)-2-amino-1,3-octadecanediol, for II;
(b) (2S,3S,4R)-2-amino-1,3,4-octadecanetriol for IV;
(c) (2S,3R,4E)-2-amino-4-octadecene-1,3-diol for sphingosine (V) (See also Lip-1.11).
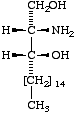 | 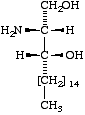 | 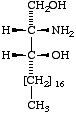 |
I
Sphinganine
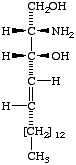
[D-erythro or 2S,3R configuration implied] | II
(2R,3R)- (or D-threo-)-2-Amino- 1,3-octadecanediol | III
Icosasphinganine (formerly eicosasphinganine,
see footnoted in Appendix A) |
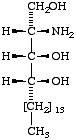 |  | 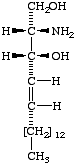 |
IV
4D-Hydroxysphinganine
(2S,3S,4R)-2-amino- 1,3,4-octadecanetriol;
(phytosphingosine). | V
Sphingosine;
(4E)-sphingenine;
trans-4-sphingenine;
(2S,3R,4E)-2-amino-4-octadecene-1,3-diol; | VI
cis-4-Sphingenine;
(4Z)-sphingenine. |
The following names have been omitted from the web version:
IV was also called 4D-Hydroxysphinganine (as above but omitted from the main web version of the lipid document).
Lip-1.8 spinganine was also called 2D-amino-1,3D-octadecanediol.
Lip-1.11 last paragraph, example (a) read: 4D-hydroxysphinganine for IV, formerly known as phytosphingosine;
References for this section
1. IUPAC-IUB Commission on Biochemical Nomenclature (1967) Eur. J. Biochem. 2, 127-131, also 12, 1 (1970); [see also Biochemistry, 6, 3287-3292 (1967); Biochem. J., 105, 897-902 (1967); J. Biol. Chem., 242, 4845-4849 (1967); Hoppe-Seyler's Z. Physiol. Chem., 350, 279-285 (1969) (in German)].
3. International Union of Biochemistry (1966) Nomenclature of Organic Chemistry (Sections A, B and C) 2nd edn, Butterworths, London. [See also Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, 1979. Edited by J Rigaudy and S P Klesney.]
4. Mills, J. A. & Klyne, W. (1954) Progr. Stereochem. 1, 181.
5. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1971) Eur. J. Biochem. 21, 455-477, also 25, 4 (1972) [now revised as Nomenclature of Carbohydrates (1996) Carb-15 is now 2-Carb-24.1].
6. International Union of Pure and Applied Chemistry (1970) J. Org. Chem. 35, 2849-2867; also Eur. J. Biochem. 18, 151-170 (1971) [now revised as Nomenclature of Organic Chemistry: Section E].
Return to main IUPAC nomenclature home page
Return to main IUBMB nomenclature home page
Return to Biochemical Nomenclature Committees home page





