https://iupac.qmul.ac.uk/cyclitol/
World Wide Web version Prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London,
E1 4NS, UK
g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version prepared by the Joint Cyclitol Nomenclature Sub-Committee - S. J. Angyal (chairman), L. Anderson, R. S. Cahn, R. M. C. Dawson, O. Hoffmann-Ostenhof, W. Klyne, and T. Posternak [see Biochem. J., 1976, 153, 23-31; Eur. J. Biochem., 1975, 57, 1-7; Pure Appl. Chem., 1974, 37, 283-297; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 149-155. Copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. A PDF of the printed version is available.
Any comments should be sent to any member of the Committee
Supplement. myo-inositol
These Recommendations are issued jointly by the Commission on the Nomenclature of Organic Chemistry of IUPAC and by the IUPAC-IUB Commission on Biochemical Nomenclature. They are the outgrowth of Tentative Rules [1] in 1968 on the basis of a report by a Joint Cyclitol Nomenclature Sub-Committee.
Scope of Cyclitol Nomenclature
Cyclitols are cycloalkanes containing one hydroxyl group on each of three or more ring atoms. These compounds, and others closely related to them, possess features of relative and absolute configuration that are characteristic of their class and have been extensively studied; but these features are not clearly displayed by general methods of stereochemical nomenclature, so that special methods of specifying their configuration are justified and have long been used. In other than stereochemical respects, their nomenclature should follow the general rules of organic chemistry.
Note
Cycloalkanes containing fewer than three hydroxyl groups are better named by the more general methods of organic chemical nomenclature.
The sequence-rule (R,S) system [2, 3] may be used when it is desired to relate a cyclitol to the general stereochemical notation and for describing the configuration of chiral groups, such as benzylidene, which may be present as substituents; but the procedures described below are recommended for cyclitol chemistry as such because of the complexity of the sequence-rule procedure in this field.
The nomenclature described below is most useful for cycloalkanes containing only one kind of substituent, and especially for cyclitols and their esters and ethers, but it may also be applied to their derivatives in which one or more hydroxyl groups have been replaced by other groups.
Evolution of Cyclitol Nomenclature
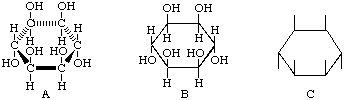
The typical stereochemical feature of cyclitols is exemplified by formula A, usually drawn more simply as B or C, in which the ring is considered as being planar and nearly perpendicular to the plane of the paper, with hydrogen atoms and hydroxyl groups above or below the plane of the ring.

In 1900, Maquenne [4] devised a fractional notation whereby numerals in the numerator denote hydroxyl or other groups (not hydrogen) above the plane of the ring while numerals in the denominator denote hydroxyl or other groups (not hydrogen) below that plane. Thus the above compound received a stereochemical prefix 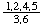 , which may be more conveniently printed as 1,2,4,5/3,6-.
, which may be more conveniently printed as 1,2,4,5/3,6-.
Maquenne did not, however, specify exactly how the numerals were to be assigned to the individual positions, and as the chemistry of cyclitols developed, these assignments were made in different ways. Several systems of nomenclature were proposed [5, 6]. Most notably, a logical and self-consistent system was developed (but not assembled as a set of rules) by Posternak [7], and his system was widely used, though with occasional variants, by others. The variety of names that resulted is illustrated in Table 1, which gives also the names derived by application of the Recommendations below.
Table 1. Examples of cyclitols named by different systems
P-R = present recommendations. P = Posternak [7]. FAL = Fletcher, Anderson and Lardy [5]. AG = Angyal and Gilman [6]
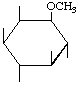 | P-R: | 1D-1-O-Methyl-myo-inositol |
| P: | 3-O-Methylmyoinositol | |
| FAL: | L-1-O-Methyl-myo-inositol | |
| AG: | (1S)-1-O-Methyl-myo-inositol | |
| Trivial name: | (-)-Bornesitol | |
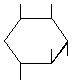 | P-R: | 1L-1,2,4/3,5-Cyclohexanepentol |
| P: | 1,2,4/3,5-Cyclohexanepentol | |
| FAL: | D-1-Deoxy-myo-inositol | |
| AG: | (1R)-vibo-Quercitol | |
| Trivial name: | (-)-Viburnitol | |
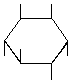 | P-R: | D-chiro-Inositol |
| P: | (+)-Chiroinositol, 1,2,5/3,4,6-inositol | |
| FAL: | D-Inositol | |
| AG: | (1S)-Inositol, (1S)-1,2,4/3,5,6-inositol | |
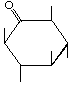 | P-R: | 2,4,6/3,5-Pentahydroxycyclohexanone |
| P: | Scyllomesoinosose, mesoinosose-2 | |
| FAL: | myo-Inosose-2 | |
| AG: | scyllo-Inosose | |
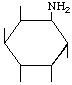 | P-R: | 1D-1-Amino-1-deoxy-neo-inositol |
| P: | Neoinosamine-3 | |
| FAL: | L-neo-lnosamine-1 | |
| AG: | (1S)-1-Amino-1-deoxy-neo-inositol | |
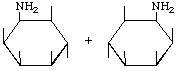 | P-R: | DL-2-Amino-2-deoxy-epi-inositol |
| P: | ( | |
| FAL: | DL-epi-Inosamine-2 | |
| AG: | ( |
An alternative method of assigning numerals, based in part on previous practice [5,6] and on proposals made by McCasland [8], was recommended by a majority of the Joint Cyclitol Nomenclature Sub-Committee, and was adopted by the parent IUPAC and IUPAC-IUB Nomenclature Commissions in 1967 [1]. By this method, enantiomers receive identical fractional prefixes that specify relative configuration, but they also receive an additional prefix D or L, which specifies the chirality.
When the Tentative Rules were published in 1968 [1], it seemed advisable to set out detailed Rules for Posternak's system because it had been widely used in the literature up to that date. These non-preferred Rules were, therefore, given in Part C of the Tentative Rules. However, this system has not been widely used during the past few years, and the 'non-preferred' Rules are therefore omitted from these Recommendations.
The present Recommendations are essentially identical with the Tentative Rules, but they have been extensively rearranged in format for the convenience of their users.
l. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1968) IUPAC Inf. Bull. 32, 51; see also Eur. J. Biochem, 5, 1 (1968); Arch. Biochem. Biophys. 128, 269 (1968); J. Biol. Chem. 243, 5809 (1968); Biochem. J. 112, 17 (1969); Biochim. Biophys. Acta, 165, 1 (1968); German language version in Hoppe-Seyler's Z. Physiol. Chem. 350, 523 (1969); French-language version in Bull. Soc. Chim. Biol. 51, 3 (1969).
2. Cahn. R. S., Ingold. C. & Prelog, V. (1966) Angew. Chem. 78, 413; Angew. Chem. Int. Ed. 5, 385.
3. International Union of Pure and Applied Chemistry (1970) J. Org. Chem. 35, 2849; see also Eur. J. Biochem. 18, 151 (1971).
4. Maquenne, L. (1900) Les sucres et leurs principaux dèrivés, Ganthiers-Villars, Paris; see also Carrè, G. & Naud, C. (1900) Paris.
5. Fletcher, H. G., Jr, Anderson, L. & Lardy, H. A. (1951) J. Org. Chem. 16, 1238.
6. Angyal, S. J. & Gilham. P. T. (1957) J. Chem. Soc. (Lond.) 3691.
7. Posternak, T. (1965) The Cyclitols, Hermann, Paris.
8. McCasland, G. E. (1965) Adv. Carbohydr. Chem. 20,11.
Return to main IUPAC Chemical Nomenclature home page
Return to main IUBMB Biochemical Nomenclature home page
Return to Biochemical Nomenclature Committees home page