Nomenclature and Symbolism for Amino Acids and Peptides
3AA-1 and 3AA-2
Contents of 3AA-1 and 3AA-2.
3AA-1 Names of Common α-Amino Acids
3AA-2 Formation of Semisystematic Names for Amino Acids and
Derivatives
References for 3AA-1 and 3AA-2
Continued in 3AA-3 to 3AA-5
Part 1. Nomenclature
Part 1, Section A: AMINO-ACID NOMENCLATURE
3AA-1. NAMES OF COMMON α-AMlNO ACIDS
The trivial names of the α-amino acids that are commonly found in proteins and are
represented in the genetic code, together with their symbols, systematic names
[14] and formulas, are given in Table 1. Some other common amino acids
are listed in the Appendix.
When the phrase 'amino acid' is a qualified noun it contains no hyphen; a hyphen is
inserted when it becomes an adjective so as to join its components in qualifying another
noun, e.g. amino-acid sequence.
Click here for "table free" view if the following
is faulty.
Table 1. α-Amino acids incorporated into protein under mRNA direction.
The systematic names and formulas given refer to hypothetical forms in which amino groups
are unprotonated and carboxyl groups are undissociated. This convention is useful to avoid
various nomenclatural problems but should not be taken to imply that these structures
represent an appreciable fraction of the amino-acid molecules.
Trivial
namea |
Symbolsb |
Systematic namec |
Formula |
| Alanine | Ala | A | 2-Aminopropanoic acid |
CH3-CH(NH2)-COOH |
| Arginine | Arg | R | 2-Amino-5-guanidinopentanoic acid |
H2N-C(=NH)-NH-[CH2]3-CH(NH2)-COOH |
| Asparagine | Asnd | N
d | 2-Amino-3-carbamoylpropanoic acid |
H2N-CO-CH2-CH(NH2)-COOH |
| Aspartic acid | Aspd | D
d | 2-Aminobutanedioic acid | HOOC-CH2-CH(NH2)-COOH |
| Cysteine | Cys | C | 2-Amino-3-mercaptopropanoic acid |
HS-CH2-CH(NH2)-COOH |
| Glutamine | Glnd | Qd | 2-Amino-4-carbamoylbutanoic acid |
H2N-CO-[CH2]2-CH(NH2)-COOH |
| Glutamic acid | Glud | E
d | 2-Aminopentanedioic acid |
HOOC-[CH2]2-CH(NH2)-COOH |
| Glycine | Gly | G | Aminoethanoic acid |
CH2(NH2)-COOH |
| Histidine | His | H | 2-Amino-3-(1H-imidazol-4-yl)-
propanoic acid |  |
| Isoleucine | Ile | I | 2-Amino-3-methylpentanoic acide |
C2H5-CH(CH3)-CH(NH2)-COOH |
| Leucine | Leu | L | 2-Amino-4-methylpentanoic acid |
(CH3)2CH-CH2-CH(NH2)-COOH |
| Lysine | Lys | K | 2,6-Diaminohexanoic acid |
H2N-[CH2]4-CH(NH2)-COOH |
| Methionine | Met | M | 2-Amino-4-(methylthio)butanoic acid |
CH3-S-[CH2]2-CH(NH2)-COOH |
| Phenylalanine | Phe | F | 2-Amino-3-phenylpropanoic acid |
C6H5-CH2-CH(NH2)-COOH |
| Proline | Pro | P | Pyrrolidine-2-carboxylic acid |
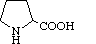 |
| Serine | Ser | S | 2-Amino-3-hydroxypropanoic acid |
HO-CH2-CH(NH2)-COOH |
| Threonine | Thr | T | 2-Amino-3-hydroxybutanoic acid
e | CH3-CH(OH)-CH(NH2)-COOH |
| Tryptophan | Trp | W | 2-Amino-3-(lH-indol-3-yl)-
propanoic acid |  |
| Tyrosine | Tyr | Y | 2-Amino-3-(4-hydroxyphenyl)-
propanoic acid | 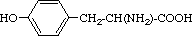 |
| Valine | Val | V | 2-Amino-3-methylbutanoic acid |
(CH3)2CH-CH(NH2)-COOH |
Unspecified amino
acid | Xaa | Xf |
a The trivial name refers to the L or D or
DL-amino acid; for those that are chiral only the L-amino acid is used for protein biosynthesis.
b Use of the one-letter symbols should be restricted to the comparison of long sequences
(3AA-20).
c The fully systematic forms ethanoic, propanoic, butanoic and pentanoic may alternatively
be called acetic, propionic, butyric and valeric, respectively. Similarly, butanedioic =
succinic, 3-carbamoylpropanoic = succinamic, pentanedioic = glutaric, and
4-carbamoylbutanoic = glutaramic.
d The symbol Asx denotes Asp or Asn; likewise B denotes N or D. Glx and Z likewise represent
glutamic acid or glutamine or a substance, such as 4-carboxyglutamic acid, Gla
(3AA-15.2.6), or 5-oxoproline, Glp (3AA-16.5), that yields glutamic acid on acid hydrolysis of
peptides.
e See 3AA-3 and -4 for stereochemical designation.
f See Addendum for alternative use of X.
g See Newsletter 1999 for use of U.
3AA-2. FORMATION OF SEMISYSTEMATIC NAMES FOR AMINO ACIDS AND DERIVATIVES
3AA-2.1. Principles of Forming Names
Semisystematic names of substituted α-amino acids are formed according to the general
principles of organic nomenclature [14], by attaching the name of the
substituent group to the trivial name of the amino acid. The position of the substitution
is indicated by locants (see 3AA-2.2). The configuration, if known,
should be indicated (see 3AA-3, 3AA-4).
New trivial names should not be coined for newly discovered α-amino acids unless
there are compelling reasons. When they are needed (e.g. because the substance is important
and its semisystematic name is cumbersome), the name should be constructed according to the
general principles for naming natural products [15], including either
some element of its chemical structure or reference to its biological origin. It is
important to use no elements in the trivial name that imply an incorrect structure; when a
new trivial name is used, it is essential that it be defined by a correctly constructed
systematic or semisystematic name. A number of existing trivial names are given in the
Appendix, and an extensive list has been published previously [6].
3AA-2.2 Designation of Locants
Note. The atom numbering given below is the normal chemical system for designating
locants. A somewhat different system has been recommended for describing polypeptide
conformations [16], in which Greek letters are used irrespective of the
nature of the atom (unless it is hydrogen), so that in lysine N-6 becomes Nζ, and in phenylalanine C-l, C-2 and C-6 become Cδ1 and Cδ2 respectively.
2.2.1. Acyclic Amino Acids
In acyclic amino acids, the carbon atom of the carboxyl group next to the carbon atom
carrying the amino group is numbered 1. Alternatively, Greek letters may be used, with C-2
being designated α. This practice is not encouraged for locants, although terms
like 'α-amino acids' and 'α-carbon atom' are retained. Example:
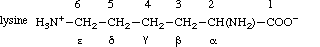 A heteroatom has the same number as the carbon atom to which it is attached, e.g. N-2 is on
C-2. When such numerals are used as locants they may be written as N6- or as 6-N, e.g. N6-acetyllysine.
A heteroatom has the same number as the carbon atom to which it is attached, e.g. N-2 is on
C-2. When such numerals are used as locants they may be written as N6- or as 6-N, e.g. N6-acetyllysine.
The carbon atoms of the methyl groups of valine are numbered 4 and 4'; likewise those of
leucine are 5 and 5'. Isoleucine is numbered as follows:
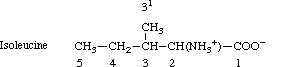 The word 'methyl' can be italicized for use as a locant for substitution on (or isotopic
modification {Section H in [14]} of) the methyl group of methionine,
e.g. [methyl-14C]methionine. The nitrogen atoms of
arginine are designated as shown for the arginine (1+)cation:
The word 'methyl' can be italicized for use as a locant for substitution on (or isotopic
modification {Section H in [14]} of) the methyl group of methionine,
e.g. [methyl-14C]methionine. The nitrogen atoms of
arginine are designated as shown for the arginine (1+)cation:
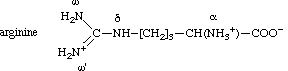 It should be noted that the ω and ω' atoms of this cation are equivalent because
of resonance. The carbon atom in the guanidino group may be called guanidino-C (it may be
needed as a locant for isotopic replacement although it cannot carry a substituent).
It should be noted that the ω and ω' atoms of this cation are equivalent because
of resonance. The carbon atom in the guanidino group may be called guanidino-C (it may be
needed as a locant for isotopic replacement although it cannot carry a substituent).
2.2.2. Proline
The carbon atoms in proline are numbered as in pyrrolidine, the nitrogen atom being
numbered 1, and proceeding towards the carboxyl group.
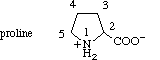 2.2.3. Aromatic Rings
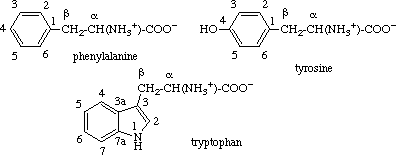
2.2.3. Aromatic Rings
The carbon atoms in the aromatic rings of phenylalanine, tyrosine and tryptophan are
numbered as in systematic nomenclature, with 1 (or 3 for tryptophan) designating the carbon
atom bearing the aliphatic chain. The carbon atoms of this chain are designated α
(for the carbon atom attached to the amino and carboxyl groups) and β (for the atom
attached to the ring system).
Note. This numbering should also be used for decarboxylated products (e.g.
tryptamine).
 2.2.4 Histidine
2.2.4 Histidine
The nitrogen atoms of the imidazole ring of histidine are denoted by pros ('near',
abbreviated π) and tele ('far', abbreviated τ) to show their position
relative to the side chain. This recommendation [6,10] arose from the fact that two different systems of numbering the atoms in the
imidazole ring of histidine had both been used for a considerable time (biochemists
generally numbering as 1 the nitrogen atom adjacent to the side chain, and organic chemists
designating it as 3). The carbon atom between the two ring nitrogen atoms is numbered 2 (as
in imidazole), and the carbon atom next to the τ nitrogen is numbered 5. The carbon
atoms of the aliphatic chain are designated α and β as in 2.2.1 and 2.2.3 above. This numbering should also be
used for the decarboxylation product histamine and for substituted histidine.
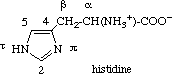 2.2.5. Definition of Side Chain
2.2.5. Definition of Side Chain
When amino acids are combined in proteins and peptides, C-l, C-2 and N-2 of each residue
(the numbering being that of aliphatic amino acids) form the repeating unit of the main
chain ('backbone') and the remainder forms a 'side chain'. Hence the words 'side chain'
refer to C-3 and higher numbered carbon atoms and their substituents.
3AA-2.3. Use of the Prefix 'homo'
An α-amino acid that is otherwise similar to one of the common ones (Table 1), but
that contains one more methylene group in the carbon chain, may be named by prefixing
'homo' to the name of that common amino acid. 'Homo' in the sense of a higher homologue
(F-4.5 of [15]) is commonly used for homoserine
(2-amino-4-hydroxybutanoic acid) and homocysteine (2-amino-4-mercaptobutanoic acid).
3AA-2.4. Use of the Prefix 'nor'
The prefix 'nor' denotes removal of a methylene group (Sections F-4.2 and F-4.4 of
[15]), but this is not the sense in which it has been used in the names
'norvaline' and 'norleucine'. Such names, although widely used, may therefore be
misinterpreted, so we cannot recommend them, especially since the systematic names for the
compounds intended, 2-aminopentanoic acid and 2-aminohexanoic acid, are short.
References
6. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB
Commission on Biochemical Nomenclature (CBN), Nomenclature of α-Amino Acids,
Recommendations 1974, Biochem. J. 149, 1-16 (1975); Biochemistry,
14, 449-462 (1975); Eur. J. Biochem. 53, 1-14 (1975); also pp.
64-77 in [7].
7. International Union of Biochemistry (1978) Biochemical Nomenclature and Related Documents, The Biochemical Society, London.
10. IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Symbols for Amino-Acid
Derivatives and Peptides, Recommendations 1971, Arch. Biochem. Biophys. 150,
1-8 (1972); Biochem. J. 126, 773-780 (1972), corrected l35, 9 (1973);
Biochemistry 11, 1726-1732 (1972); Biochim. Biophys. Acta, 263,
205-212 (1972); Eur. J. Biochem. 27, 201-207 (1972), corrected 45, 2
(1974); J. Biol. Chem. 247, 977-983 (1972); Pure Appl. Chem. 40,
315-331 (1974); also pp. 78-84 in [7].
14. International Union of Pure and Applied Chemistry (1979) Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, Pergamon Press, Oxford.
15. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC). Nomenclature of
Organic Chemistry, Section F: Natural Products and Related Compounds, Recommendations 1976,
Eur. J. Biochem. 86, 1-8 (1978); also pp. 19-26 in [7]
and pp. 491-511 in [14]. [See also Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 19-26.]
16. IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Abbreviations and Symbols for the Description of the Conformation of Polypeptide Chains, 1969, Arch. Biochem. Biophys. 145, 405-421 (1971); Biochem. J. 121, 577-585 (1971); Biochemistry, 9, 3471-3479 (1970); Biochim. Biophys. Acta, 229, 1-17 (1971); Eur. J. Biochem. 17, 193-201 (1970); J. Biol. Chem. 245, 6489-6497 (1970); Mol. Biol. 7, 289-303 (1973) (in Russian); Pure Appl. Chem. 40, 291-308 (1974); also pp. 94-102 in [7].
Continue to the next section with 3AA-3 to 3AA-5 of Amino Acids
and Peptides.
Return to Amino Acids and Peptides home page.