Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-25 and 2-Carb-26
Continued from 2-Carb-24
Contents
2-Carb-25. N-Substitution
Substitution, e.g. acylation, at the NH2 group of an amino sugar can be dealt with in two different ways:
(a) The whole substituted amino group can be designated as a prefix, e.g. 2-acetamido- (or 2- butylamino-) 2-deoxy-D-glucose. For the purpose of the configurational prefix, the group is considered to take the place of the former OH group.
(b) If the amino sugar has a trivial name, the substitution is indicated by a prefix preceded by an italic capital N.
Note. In carbohydrate nomenclature, substitution at a heteroatom is normally indicated by citing the locant of the attached carbon atom, followed by a hyphen, and then the italicized heteroatom element symbol, e.g. 2-O-methyl, 5-N-acetyl. Substituents on the same kind of heteroatom are grouped (e.g. 2,3,4-tri-O-methyl), and substituents of the same kind are cited in alphabetical order of heteroatoms (e.g. 5-N-acetyl-4,8,9-tri-O-acetyl). The alternative format with superscript numerical locants (e.g. N5,O4,O8,O9-tetraacetyl), used in some other areas of natural product chemistry, is unusual in carbohydrate names.
Examples:
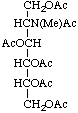
1,3,4,5,6-Penta-O-acetyl-2-deoxy-2-(N-methylacetamido)-D-glucitol
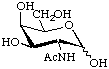
2-Acetamido-2-deoxy-D-galactopyranose
or N-acetyl-D-galactosamine
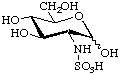
2-Deoxy-2-sulfoamino-D-glucopyranose
or N-sulfo-D-glucosamine
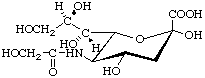
N-Glycoloyl-α-neuraminic acid (α-Neu5Gc) (D is implied in the trivial name)
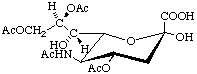
5-N-Acetyl-4,8,9-tri-O-acetyl-α-neuraminic acid (α-Neu4,5,8,9Ac4)
2-Carb-26. Intramolecular anhydrides
An intramolecular ether (commonly called an intramolecular anhydride), formally arising by elimination of water from two hydroxy groups of a single molecule of a monosaccharide (aldose or ketose) or monosaccharide derivative, is named by attaching the (detachable) prefix 'anhydro-' preceded by a pair of locants identifying the two hydroxy groups involved.
Note. Detachable prefixes are cited in alphabetical order along with any substituent prefixes.
Examples:
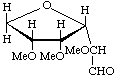
3,6-Anhydro-2,4,5-tri-O-methyl-D-glucose

2,5-Anhydro-D-allononitrile
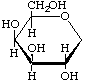
1,5-Anhydro-D-galactitol
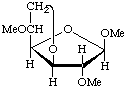
Methyl 3,6-anhydro-2,5-di-O-methyl-β-D-glucofuranoside
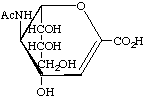
5-Acetamido-2,6-anhydro-3,5-dideoxy-D-glycero-D-galacto-non-2-enonic acid (Neu2en5Ac)
The compounds usually known as monosaccharide anhydrides or glycose anhydrides (earlier 'glycosans'), formation of which involves the anomeric hydroxy group, are named by the same procedure. In these cases the order of preference of ring size designators is pyranose > furanose > septanose. However, three- or four-membered rings should normally be cited as 'anhydro' if there is a choice.
Trivial names for anhydro monosaccharides, though established by usage, are not recommended because of possible confusion with polysaccharide names based on the use of the termination '-an'.
Examples:
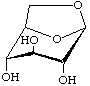
1,6-Anhydro-β-D-glucopyranose
not 1,5-anhydro-α-D-glucoseptanose
(older trivial name: levoglucosan)
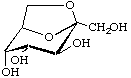
2,7-Anhydro-β-D-altro-hept-2-ulopyranose
(older trivial name: sedoheptulosan)
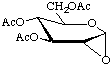
3,4,6-Tri-O-acetyl-1,2-anhydro-α-D-glucopyranose
not 3,4,6-tri-O-acetyl-1,5-anhydro-β-D-glucooxirose
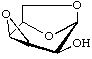
1,6:3,4-Dianhydro-β-D-talopyranose
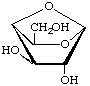
1,4-Anhydro-β-D-galactopyranose
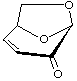
1,6-Anhydro-3,4-dideoxy-β-D-glycero-hex-3-enopyranos-2-ulose
(trivial name: levoglucosenone)
Continue to the next section with 2-Carb-27 and 2-Carb-28 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.